Korean J Radiol.
2020 Mar;21(3):280-289. 10.3348/kjr.2019.0447.
Characterizing Computed Tomography-Detected Arterial Hyperenhancing-Only Lesions in Patients at Risk of Hepatocellular Carcinoma: Can Non-Contrast Magnetic Resonance Imaging Be Used for Sequential Imaging?
- Affiliations
-
- 1Department of Radiology, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea.
- 2Department of Radiology, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea. kbh@ajou.ac.kr
- 3Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2470751
- DOI: http://doi.org/10.3348/kjr.2019.0447
Abstract
OBJECTIVE
To test the feasibility of non-contrast magnetic resonance imaging (MRI) in a sequential imaging study for characterizing computed tomography (CT)-detected arterial-enhancing nodules that do not washout in patients at risk of hepatocellular carcinoma (HCC).
MATERIALS AND METHODS
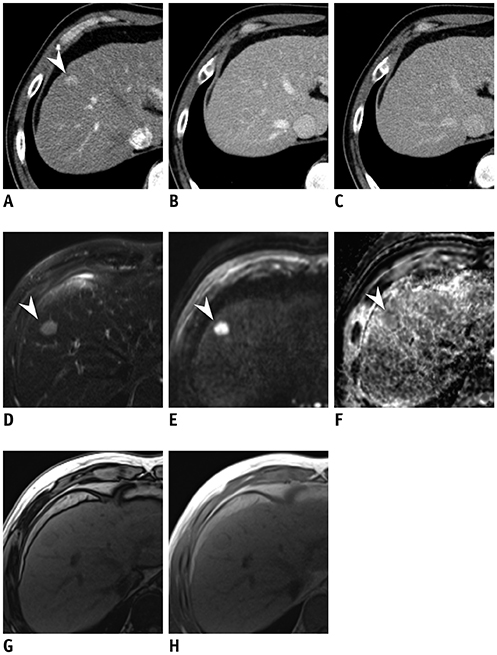
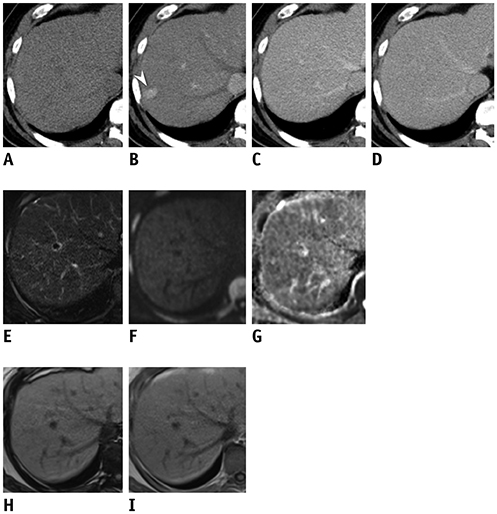
In this retrospective study, 134 patients (mean age ± standard deviation, 56.8 ± 10.0 years) with 151 arterial enhancing-only nodules measuring up to 2 cm during multiphasic CT that were subsequently evaluated using gadoxetic acid-enhanced MRI in treatment-naïve at-risk patients from three tertiary referral centers were included. Tentative diagnostic criteria for HCC and hepatic malignancy were defined as the presence of one of eight MRI features favoring HCC in combinations of the following sequences: T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), T1-weighted dual gradient-echo in-phase and out-of-phase imaging (Dual-GRE), and hepatobiliary phase imaging (HBP). Typical hemangiomas and arterioportal shunts were excluded from the analysis. Diagnostic performance for HCC and hepatic malignancy was calculated and compared between the abbreviated MRI and full-sequence gadoxetic acid-enhanced MRI.
RESULTS
Of 151 nodules (mean size, 1.2 cm) 68 HCCs and 83 non-HCC benignities and malignancies were included. The combination of T2WI, DWI, and Dual-GRE showed per-lesion sensitivity, specificity, and accuracy of 88.2%, 90.4%, and 89.4%, respectively, comparable to those of full-sequence MRI. Applying the same sequence combination to diagnose hepatic malignancy had per-lesion sensitivity, specificity, and accuracy of 86.8%, 97.3%, and 92.1%. In nodules < 1 cm, adding HBP increased sensitivity by up to 13% without compromising the specificity or accuracy.
CONCLUSION
The non-contrast MRI protocol comprising T2WI, DWI, and Dual-GRE showed reasonable and comparable performance to full-sequence MRI for discriminating HCC and primary liver malignancies in CT-detected indeterminate arterial enhancing-only nodules in at-risk patients, and can be potentially used for sequential imaging in place of a full-sequence MRI. In nodules < 1 cm, HBP may still be needed to preserve sensitivity.
MeSH Terms
Figure
Reference
-
1. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.
Article2. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.3. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017; 11:317–370.
Article4. CT/MRI LI-RADS® v2018 CORE. American College of Radiology. 2018. Accessed June 20, 2019. Available at: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf?la=en.5. Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC). 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2019; 20:1042–1113.6. Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011; 29:339–364.
Article7. Choi BI, Lee KH, Han JK, Lee JM. Hepatic arterioportal shunts: dynamic CT and MR features. Korean J Radiol. 2002; 3:1–15.
Article8. Sherman M. Diagnosis of small hepatocellular carcinoma. Hepatology. 2005; 42:14–16.
Article9. Aubé C, Oberti F, Lonjon J, Pageaux G, Seror O, N'Kontchou G, et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int. 2017; 37:1515–1525.
Article10. Kim SH, Kim SH, Lee J, Kim MJ, Jeon YH, Park Y, et al. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2009; 192:1675–1681.
Article11. Liu X, Jiang H, Chen J, Zhou Y, Huang Z, Song B. Gadoxetic acid disodium-enhanced magnetic resonance imaging outperformed multidetector computed tomography in diagnosing small hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2017; 23:1505–1518.
Article12. Motosugi U, Ichikawa T, Sou H, Sano K, Tominaga L, Muhi A, et al. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology. 2010; 256:151–158.
Article13. Park SH, Kim B, Kim SY, Shim YS, Kim JH, Huh J, et al. Abbreviated MRI with optional multiphasic CT as an alternative to full-sequence MRI: LI-RADS validation in a HCC-screening cohort. Eur Radiol. 2019; 12. 19. [Epub ahead of print]. DOI: 10.1007/s00330-019-06546-5.
Article14. Choi SH, Byun JH, Lim YS, Yu E, Lee SJ, Kim SY, et al. Diagnostic criteria for hepatocellular carcinoma ≤ 3 cm with hepatocyte-specific contrast-enhanced magnetic resonance imaging. J Hepatol. 2016; 64:1099–1107.15. Kim TK, Lee KH, Jang HJ, Haider MA, Jacks LM, Menezes RJ, et al. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1–2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology. 2011; 259:730–738.
Article16. Piana G, Trinquart L, Meskine N, Barrau V, Beers BV, Vilgrain V. New MR imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Hepatol. 2011; 55:126–132.
Article17. Campos JT, Sirlin CB, Choi JY. Focal hepatic lesions in Gd-EOB-DTPA enhanced MRI: the atlas. Insights Imaging. 2012; 3:451–474.
Article18. Duncan JK, Ma N, Vreugdenburg TD, Cameron AL, Maddern G. Gadoxetic acid-enhanced MRI for the characterization of hepatocellular carcinoma: a systematic review and meta-analysis. J Magn Reson Imaging. 2017; 45:281–290.
Article19. Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol. 2017; 3:456–463.
Article20. Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology. 2018; 67:401–421.
Article21. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014; 273:30–35.
Article22. Joo I, Lee JM, Lee DH, Jeon JH, Han JK, Choi BI. Noninvasive diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout? Eur Radiol. 2015; 25:2859–2868.
Article23. Kierans AS, Kang SK, Rosenkrantz AB. The diagnostic performance of dynamic contrast-enhanced MR imaging for detection of small hepatocellular carcinoma measuring up to 2 cm: a meta-analysis. Radiology. 2016; 278:82–94.
Article24. Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology. 2010; 255:459–466.
Article25. Sirlin C. Use of the liver imaging reporting and data system in hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2017; 13:363–336.26. Chang KJ, Kamel IR, Macura KJ, Bluemke DA. 3.0-T MR imaging of the abdomen: comparison with 1.5 T. Radiographics. 2008; 28:1983–1998.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent development of diagnostic imaging of hepatocellular carcinoma
- A study on the comparision of various imaging methods for the staging of renal cell carcinoma
- A Comprehensive Review of Hepatocellular Carcinoma Enhancement Patterns in MRI: Emphasis on Gadoxetate-Enhanced Imaging
- Unraveling distinctions between contrast-enhanced ultrasound and CT/MRI for liver mass diagnosis
- Local ablation therapy with contrast-enhanced ultrasonography for hepatocellular carcinoma: a practical review