Korean J Ophthalmol.
2020 Feb;34(1):56-66. 10.3341/kjo.2019.0112.
Hierarchical Cluster Analysis of Peripapillary Retinal Nerve Fiber Layer Damage and Macular Ganglion Cell Loss in Open Angle Glaucoma
- Affiliations
-
- 1Institute of Vision Research, Department of Ophthalmology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. kcyeye@yuhs.ac
- KMID: 2469287
- DOI: http://doi.org/10.3341/kjo.2019.0112
Abstract
- PURPOSE
To categorize the structural progression pattern of glaucoma, as detected by optical coherence tomography guided progression analysis, with respect to the peripapillary retinal nerve fiber layer (RNFL) and macular ganglion cell-inner plexiform layer (GCIPL).
METHODS
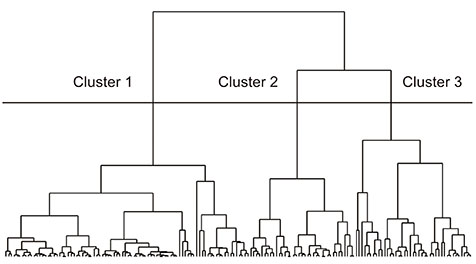
One hundred sixty-four eyes with primary open-angle glaucoma were studied. The structural progression pattern evaluated by optical coherence tomography guided progression analysis was classified using hierarchical cluster analysis. The clinical parameters, patterns of structural progression, and visual field (VF) changes were compared among the groups.
RESULTS
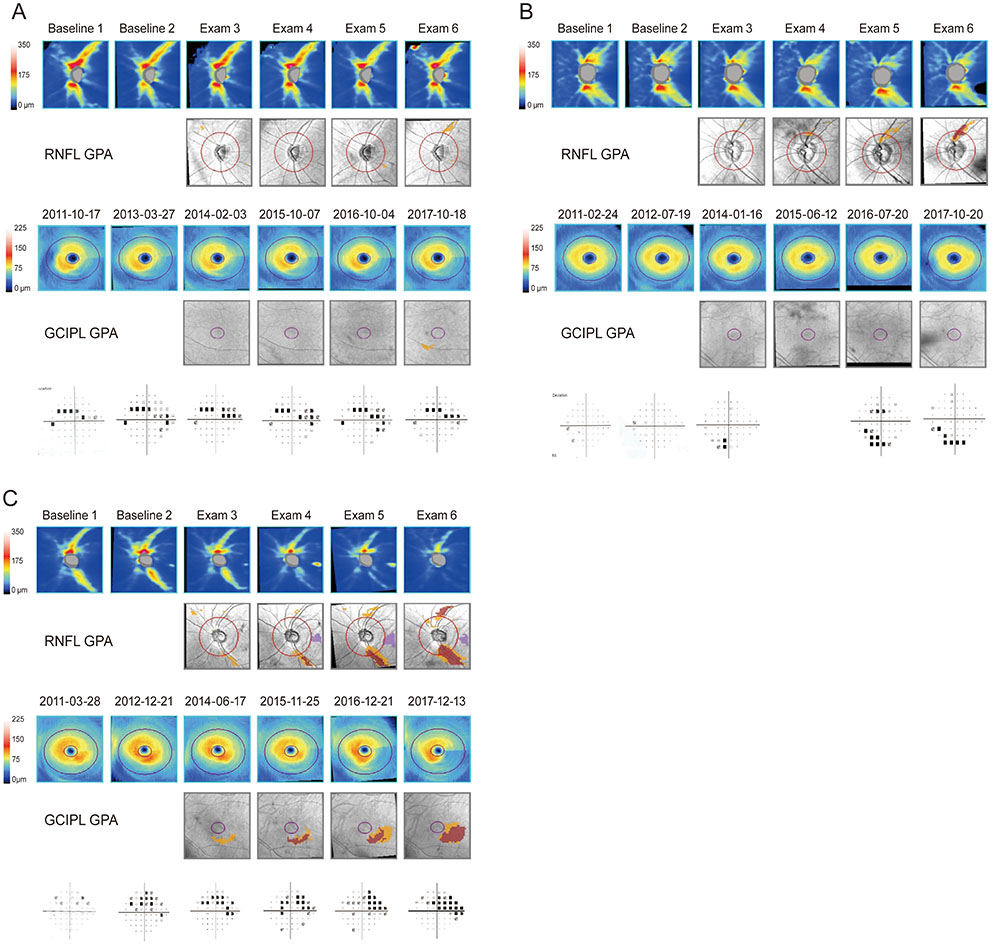
Three groups were included: stable, progressive peripapillary RNFL thinning without macular GCIPL involvement, and progressive thinning of both the peripapillary RNFL and macular GCIPL. The third group, those with progressive peripapillary RNFL and macular GCIPL thinning, showed more progressive peripapillary RNFL thinning in the inferotemporal area and VF progression in the parafoveal area. Conversely, the 12 and 6 o'clock areas were the most common locations of progressive peripapillary RNFL thinning in the group without macular GCIPL involvement.
CONCLUSIONS
Structural progression patterns of glaucoma can be categorized into three groups. The location of progressive peripapillary RNFL thinning is associated with progressive macular GCIPL thinning and pattern of VF changes in the affected area. Our results indicate that the use of only macular GCIPL analysis is inadequate for analyzing the structural progression of glaucoma.
MeSH Terms
Figure
Reference
-
1. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004; 363:1711–1720.
Article2. Casson RJ, Chidlow G, Wood JP, et al. Definition of glaucoma: clinical and experimental concepts. Clin Exp Ophthalmol. 2012; 40:341–349.
Article3. Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007; 26:688–710.
Article4. Kerrigan-Baumrind LA, Quigley HA, Pease ME, et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000; 41:741–748.5. Artes PH, Chauhan BC. Longitudinal changes in the visual field and optic disc in glaucoma. Prog Retin Eye Res. 2005; 24:333–354.
Article6. Chauhan BC, McCormick TA, Nicolela MT, LeBlanc RP. Optic disc and visual field changes in a prospective longitudinal study of patients with glaucoma: comparison of scanning laser tomography with conventional perimetry and optic disc photography. Arch Ophthalmol. 2001; 119:1492–1499.7. Alencar LM, Zangwill LM, Weinreb RN, et al. Agreement for detecting glaucoma progression with the GDx guided progression analysis, automated perimetry, and optic disc photography. Ophthalmology. 2010; 117:462–470.
Article8. Xin D, Greenstein VC, Ritch R, et al. A comparison of functional and structural measures for identifying progression of glaucoma. Invest Ophthalmol Vis Sci. 2011; 52:519–526.
Article9. Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991; 109:77–83.
Article10. Harwerth RS, Carter-Dawson L, Shen F, et al. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999; 40:2242–2250.11. Nouri-Mahdavi K, Nowroozizadeh S, Nassiri N, et al. Macular ganglion cell/inner plexiform layer measurements by spectral domain optical coherence tomography for detection of early glaucoma and comparison to retinal nerve fiber layer measurements. Am J Ophthalmol. 2013; 156:1297–1307.
Article12. Mwanza JC, Durbin MK, Budenz DL, et al. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology. 2012; 119:1151–1158.
Article13. Na JH, Sung KR, Lee JR, et al. Detection of glaucomatous progression by spectral-domain optical coherence tomography. Ophthalmology. 2013; 120:1388–1395.
Article14. Kim KE, Yoo BW, Jeoung JW, Park KH. Long-term reproducibility of macular ganglion cell analysis in clinically stable glaucoma patients. Invest Ophthalmol Vis Sci. 2015; 56:4857–4864.
Article15. Kim NR, Lee ES, Seong GJ, et al. Comparing the ganglion cell complex and retinal nerve fibre layer measurements by Fourier domain OCT to detect glaucoma in high myopia. Br J Ophthalmol. 2011; 95:1115–1121.
Article16. Kim YK, Ha A, Na KI, et al. Temporal relation between macular ganglion cell-inner plexiform layer loss and peripapillary retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2017; 124:1056–1064.
Article17. Chiang DY, Brown PO, Eisen MB. Visualizing associations between genome sequences and gene expression data using genome-mean expression prof iles. Bioinformatics. 2001; 17:S49–S55.18. Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998; 95:14863–14868.
Article19. Liu Y, Hayes DN, Nobel A, Marron JS. Statistical significance of clustering for high-dimension, low-sample size data. J Am Stat Assoc. 2008; 103:1281–1293.
Article20. Shin JW, Sung KR, Lee GC, et al. Ganglion cell-inner plexiform layer change detected by optical coherence tomography indicates progression in advanced glaucoma. Ophthalmology. 2017; 124:1466–1474.
Article21. Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007; 114:1965–1972.
Article22. Ward JH Jr. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963; 58:236–244.
Article23. Charrad M, Ghazzali N, Boiteau V, et al. Package ‘nbclust’. J Stat Softw. 2014; 61:1–36.24. Duda RO, Hart PE, Stork DG. Pattern classif ication. Hoboken: John Wiley & Sons;2012.25. Hodapp E, Parrish RK, Anderson DR. Clinical decisions in glaucoma. Maryland Heights: Mosby;1993.26. Shin HY, Park HL, Jung KI, et al. Glaucoma d iagnostic ability of ganglion cell-inner plexiform layer thickness differs according to the location of visual field loss. Ophthalmology. 2014; 121:93–99.27. Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. Br J Ophthalmol. 2003; 87:899–901.
Article28. Lee EJ, Kim TW, Weinreb RN, et al. Trend-based analysis of retinal nerve fiber layer thickness measured by optical coherence tomography in eyes with localized nerve fiber layer defects. Invest Ophthalmol Vis Sci. 2011; 52:1138–1144.
Article29. Leung CKS, Ye C, Weinreb RN, et al. Impact of age-related change of retinal nerve fiber layer and macular thicknesses on evaluation of glaucoma progression. Ophthalmology. 2013; 120:2485–2492.
Article30. Kim YK, Jeoung JW, Park KH. Inferior macular damage in glaucoma: its relationship to retinal nerve fiber layer defect in macular vulnerability zone. J Glaucoma. 2017; 26:126–132.
Article31. Hwang YH, Jeong YC, Kim HK, Sohn YH. Macular ganglion cell analysis for early detection of glaucoma. Ophthalmology. 2014; 121:1508–1515.
Article32. Wollstein G, Kagemann L, Bilonick RA, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol. 2012; 96:47–52.
Article33. Lee EJ, Kim TW, Weinreb RN, et al. β-Zone parapapillary atrophy and the rate of retinal nerve fiber layer thinning in glaucoma. Invest Ophthalmol Vis Sci. 2011; 52:4422–4427.
Article34. Mwanza JC, Chang RT, Budenz DL, et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010; 51:5724–5730.
Article35. Wang X, Jiang C, Ko T, et al. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2015; 253:1557–1564.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Evaluation of Unilateral Open-Angle Glaucoma: A Two-Year Follow-Up Study
- Correlation Between Macular, Retinal Nerve Fiber Layer Thickness, and Visual Field in Open Angle Glaucoma
- Analysis of Choroidal Thickness Measured Using RTVue and Associated Factors in Open-Angle Glaucoma
- Associations of Peripapillary Retinal Nerve Fiber Layer and Macular Retinal Layer Thickness with Serum Homocysteine Concentration
- Effect of ERM on RNFL, GCIPL and Macular Thickness According to the Severity of Glaucoma