J Korean Med Sci.
2016 Sep;31(9):1383-1391. 10.3346/jkms.2016.31.9.1383.
The Immunogenicity and Safety of a Combined DTaP-IPV//Hib Vaccine Compared with Individual DTaP-IPV and Hib (PRP~T) Vaccines: a Randomized Clinical Trial in South Korean Infants
- Affiliations
-
- 1Department of Pediatrics, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- 2Department of Pediatrics, Seoul National University Children's Hospital, Seoul National University College of Medicine, Seoul, Korea. hoanlee@snu.ac.kr
- 3Department of Pediatrics, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- 4Department of Pediatrics, Hanyang University Medical Center, Seoul, Korea.
- 5Department of Pediatrics, Kyung Hee University Hospital, Seoul, Korea.
- 6Department of Pediatrics, KEPCO Medical Center, Seoul, Korea.
- 7Department of Pediatrics, Inje University Ilsan Paik Hospital, Ilsan, Korea.
- 8Department of Pediatrics, Eulji University School of Medicine, Eulji General Hospital, Seoul, Korea.
- 9Department of Pediatrics, Soonchunhyang University Bucheon Hospital, Bucheon, Korea.
- 10Department of Pediatrics, Inha University Hospital, Incheon, Korea.
- 11Department of Pediatrics, The Catholic University of Korea, Uijeongbu St. Mary's Hospital, Uijeongbu, Korea.
- 12Department of Pediatrics, The Catholic University of Korea, Daejeon St. Mary's Hospital, Daejeon, Korea.
- 13Department of Pediatrics, Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, Korea.
- 14Sanofi Pasteur, Seoul, Korea.
- 15Sanofi Pasteur, Lyon, France.
- KMID: 2468269
- DOI: http://doi.org/10.3346/jkms.2016.31.9.1383
Abstract
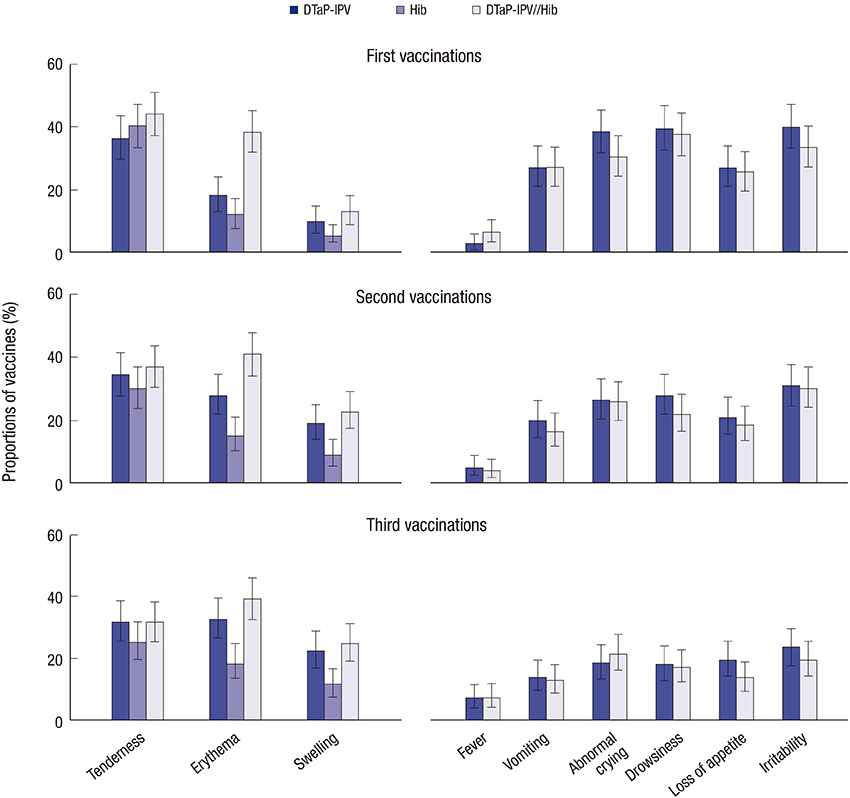
- Recommended infant vaccination in Korea includes DTaP-IPV and Hib vaccines administered as separate injections. In this randomized, open, controlled study we assessed the non-inferiority of immunogenicity of DTaP-IPV//Hib pentavalent combination vaccine (Pentaximâ„¢) compared with licensed DTaP-IPV and Hib (PRP~T) vaccines. We enrolled 418 healthy Korean infants to receive either separate DTaP-IPV and Hib vaccines (n = 206) or the pentavalent DTaP-IPV//Hib (n = 208) vaccine at 2, 4, 6 months of age. Antibodies to all components were measured before the first vaccination and one month after the third, and safety was assessed after each vaccination including recording of reactions by parents. We confirmed the non-inferiority of DTaP-IPV//Hib compared with DTaP-IPV and Hib vaccines; 100% of both groups achieved seroprotection against D, T, IPV and PRP~T, and 97.5%-99.0% demonstrated seroresponses to pertussis antigens. Antibody levels were similar in both groups, except for those to the Hib component, PRP~T. In separate and combined groups geometric mean concentrations of anti-PRP~T antibodies were 23.9 and 11.0 µg/mL, respectively, but 98.3% and 97.4% had titers ≥ 1 µg/mL, indicative of long-term protection. All vaccines were well tolerated, with no vaccine-related serious adverse event. Both groups had similar safety profiles, but the combined vaccine group had fewer injection site reactions. The immunological non-inferiority and similar safety profile of DTaP-IPV//Hib vaccine to separate DTaP-IPV and Hib vaccines, with the advantage of fewer injections and injection site reactions, supports the licensure and incorporation of DTaP-IPV//Hib into the Korean national vaccination schedule (Clinical trial registry, NCT01214889).
Keyword
MeSH Terms
-
Antibodies, Bacterial/blood
Asian Continental Ancestry Group
Diphtheria-Tetanus-Pertussis Vaccine/*immunology
Enzyme-Linked Immunosorbent Assay
Female
Haemophilus Vaccines/*immunology
Haemophilus influenzae type b/immunology
Humans
Immunization Schedule
Infant
Male
Poliovirus Vaccine, Inactivated/*immunology
Republic of Korea
Tetanus Toxoid/*immunology
Vaccines, Combined/immunology
Vaccines, Conjugate/immunology
Antibodies, Bacterial
Diphtheria-Tetanus-Pertussis Vaccine
Haemophilus Vaccines
Poliovirus Vaccine
Tetanus Toxoid
Vaccines, Combined
Vaccines, Conjugate
Figure
Reference
-
1. World Health Organization. WHO recommendations for routine immunizations - summary tables. accessed on 17 July 2015. Available at http://www.who.int/immunization/policy/immunization_schedules/en/.2. Vidor E, Plotkin SA. Immunogenicity of a two-component (PT & FHA) acellular pertussis vaccine in various combinations. Hum Vaccin. 2008; 4:328–340.3. Plotkin SA, Liese J, Madhi SA, Ortiz EA. DTaP-IPV//PRP~T vaccine (Pentaxim): a review of 16 years' clinical experience. Expert Rev Vaccines. 2011; 10:981–1005.4. Eskola J, Ward J, Dagan R, Goldblatt D, Zepp F, Siegrist CA. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet. 1999; 354:2063–2068.5. Collins S, Ramsay M, Campbell H, Slack MP, Ladhani SN. Invasive Haemophilus influenzae type b disease in England and Wales: who is at risk after 2 decades of routine childhood vaccination? Clin Infect Dis. 2013; 57:1715–1721.6. World Health Organization. WHO position paper on Haemophilus influenzae type b vaccines. Wkly Epidemiol Rec. 2006; 81:444–452.7. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998; 17:857–872.8. Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998; 17:873–890.9. Lee H, Hahn S, Lee HJ, Kim KH. Immunogenicity of Haemophilus influenzae type b conjugate vaccines in Korean infants: a meta-analysis. J Korean Med Sci. 2010; 25:90–96.10. Käyhty H, Peltola H, Karanko V, Mäkelä PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983; 147:1100.11. Cherry JD. Update on pertussis and diphtheria-tetanus toxoids-pertussis vaccination new strategies for clinicians. Introduction. Pediatr Infect Dis J. 1997; 16:S76–7.12. Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi AE, Anemona A. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. N Engl J Med. 1996; 334:341–348.13. Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med. 1996; 334:349–355.14. Gzyl A, Augustynowicz E, Rabczenko D, Gniadek G, Ślusarczyk J. Potency of pertussis component in the DTP vaccine--an overview of three decade study in Poland. Biologicals. 2004; 32:129–137.15. Thollot F, Scheifele D, Pankow-Culot H, Cheuvart B, Leyssen M, Ulianov L, Miller JM. A randomized study to evaluate the immunogenicity and safety of a heptavalent diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis, Haemophilus influenzae b, and meningococcal serogroup C combination vaccine administered to infants at 2, 4 and 12 months of age. Pediatr Infect Dis J. 2014; 33:1246–1254.16. Kim JS, Cho SB, Lee HR, Park SK, Hwang PH. Immunogenicity and safety of Haemophilus influenzae type b polysaccharide-tetanus toxoid conjugate vaccine (PRP-T) in Korean infants. Korean J Infect Dis. 1996; 28:225–232.17. Lee SY, Hwang HS, Kim JH, Kim HH, Lee HS, Chung EH, Park SE, Ma SH, Chang JK, Guitton F, et al. Immunogenicity and safety of a combined diphtheria, tetanus, acellular pertussis, and inactivated poliovirus vaccine (DTaP-IPV) compared to separate administration of standalone DTaP and IPV vaccines: a randomized, controlled study in infants in the Republic of Korea. Vaccine. 2011; 29:1551–1557.18. Peltola H, Aavitsland P, Hansen KG, Jónsdóttir KE, Nøkleby H, Romanus V. Perspective: a five-country analysis of the impact of four different Haemophilus influenzae type b conjugates and vaccination strategies in Scandinavia. J Infect Dis. 1999; 179:223–229.19. Georges S, Lepoutre A, Dabernat H, Levy-Bruhl D. Impact of Haemophilus influenzae type b vaccination on the incidence of invasive Haemophilus influenzae disease in France, 15 years after its introduction. Epidemiol Infect. 2013; 141:1787–1796.20. Kim KH, Kim YK, Kim NH, Chang SH, Lee J, Park EA, Park SE, Eun BW, Lee H, Lee HJ. Immunogenicity and safety of LBVH0101, a new Haemophilus influenzae type b tetanus toxoid conjugate vaccine, compared with Hiberix™ in Korean infants and children: a randomized trial. Vaccine. 2012; 30:1886–1894.21. Bedford H, Lansley M. More vaccines for children? Parents' views. Vaccine. 2007; 25:7818–7823.22. Wallace AS, Mantel C, Mayers G, Mansoor O, Gindler JS, Hyde TB. Experiences with provider and parental attitudes and practices regarding the administration of multiple injections during infant vaccination visits: lessons for vaccine introduction. Vaccine. 2014; 32:5301–5310.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity and Safety of Two Different Haemophilus influenzae Type b Conjugate Vaccines in Korean Infants
- Present status and prospects of Haemophilus influenzae type b(Hib) immunization
- The immunogenicity and safety of three-component DTaP vaccine in Korean infants
- Recommendation for use of diphtheria and tetanus toxoids and acellular pertussis, inactivated poliovirus, Haemophilus influenzae type b conjugate, and hepatitis B vaccine in infants
- Immunogenicity of Haemophilus influenzae PRP-D Conjugate Vaccine in Korean Infants