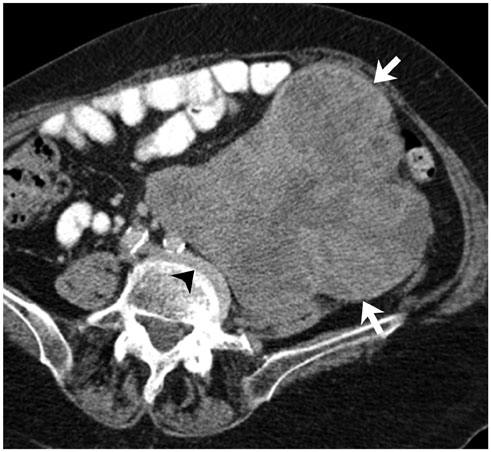
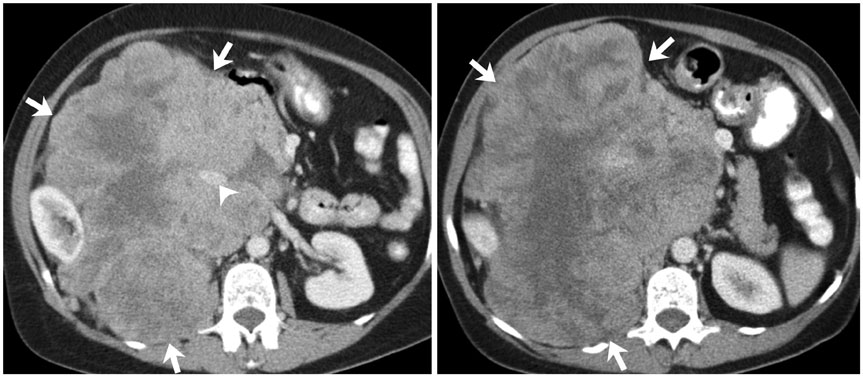
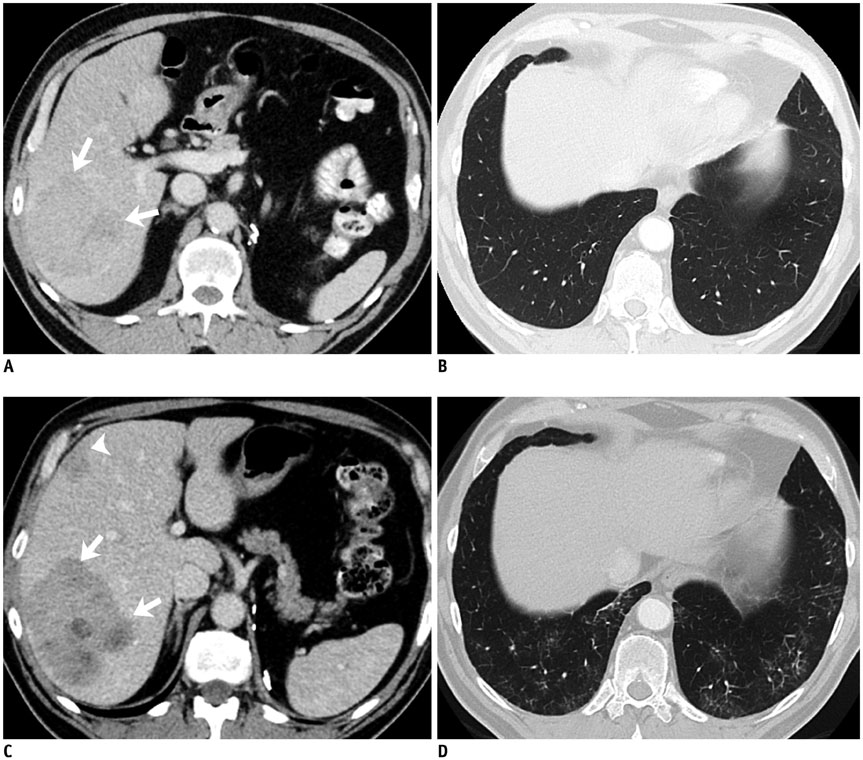
Korean J Radiol.
2017 Feb;18(1):94-106. 10.3348/kjr.2017.18.1.94.
Current Concepts in Non-Gastrointestinal Stromal Tumor Soft Tissue Sarcomas: A Primer for Radiologists
- Affiliations
-
- 1Department of Radiology, Tata Memorial Centre, Mumbai 400012, India. akshaybaheti@gmail.com
- 2Department of Radiology, Brigham and Women's Hospital, Boston, MA 02115, USA.
- 3Department of Imaging, Dana-Farber Cancer Institute, Boston, MA 02215, USA.
- 4Department of Radiology, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
- KMID: 2468125
- DOI: http://doi.org/10.3348/kjr.2017.18.1.94
Abstract
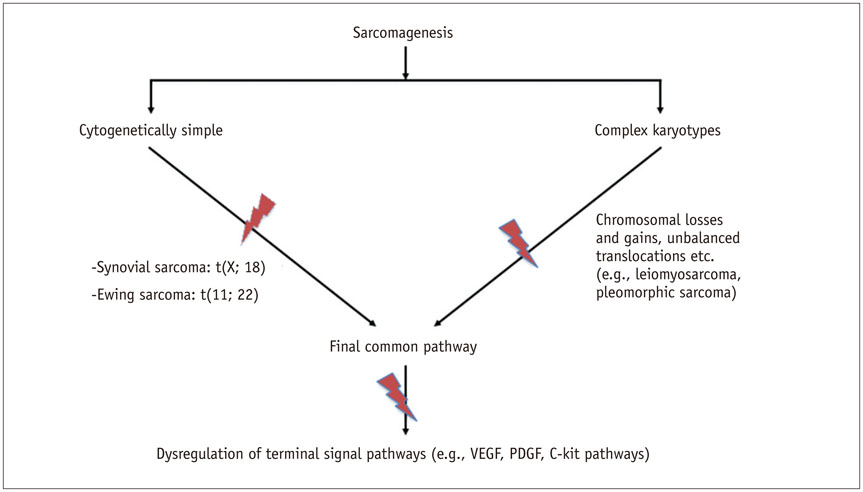
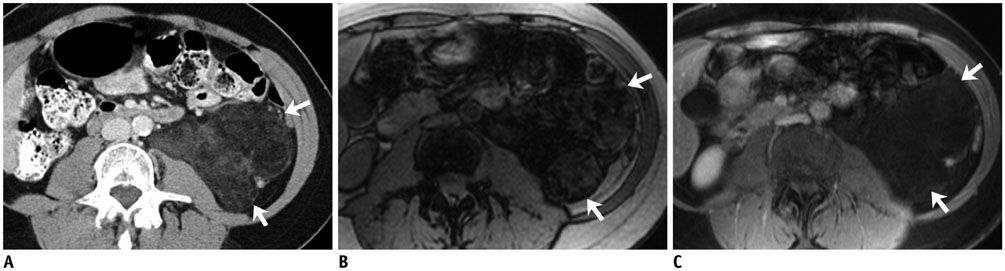
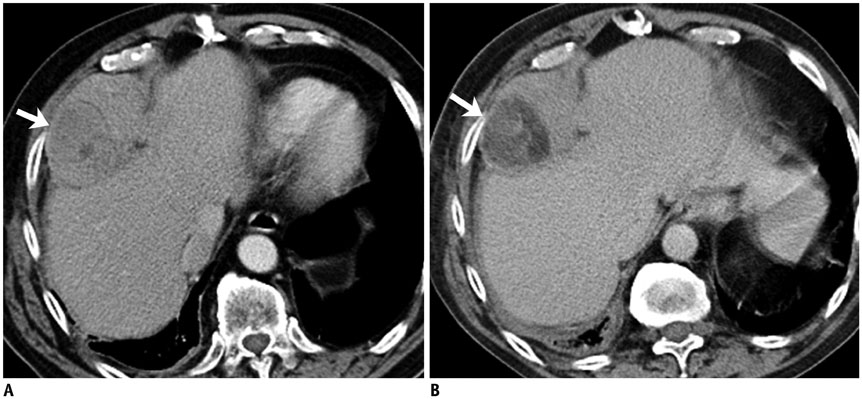
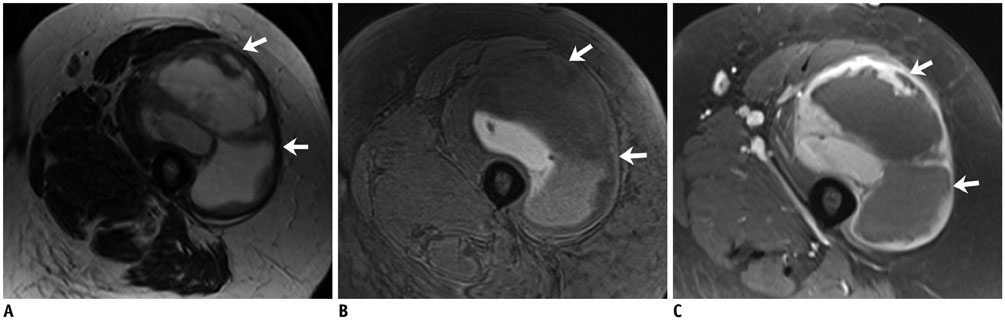
- Non-gastrointestinal stromal tumor (GIST) soft tissue sarcomas (STSs) are a heterogeneous group of neoplasms whose classification and management continues to evolve with better understanding of their biologic behavior. The 2013 World Health Organization (WHO) has revised their classification based on new immunohistochemical and cytogenetic data. In this article, we will provide a brief overview of the revised WHO classification of soft tissue tumors, discuss in detail the radiology and management of the two most common adult non-GIST STS, namely liposarcoma and leiomyosarcoma, and review some of the emerging histology-driven targeted therapies in non-GIST STS, focusing on the role of the radiologist.
MeSH Terms
Figure
Reference
-
1. Baheti AD, O'Malley RB, Kim S, Keraliya AR, Tirumani SH, Ramaiya NH, et al. Soft-tissue sarcomas: an update for radiologists based on the revised 2013 World Health Organization classification. AJR Am J Roentgenol. 2016; 206:924–932.2. Ducimetière F, Lurkin A, Ranchère-Vince D, Decouvelaere AV, Pèoc’h M, Istier L, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011; 6:e20294.3. Tirumani SH, Jagannathan JP, Krajewski KM, Shinagare AB, Jacene H, Ramaiya NH. Imatinib and beyond in gastrointestinal stromal tumors: a radiologist's perspective. AJR Am J Roentgenol. 2013; 201:801–810.4. Tirumani SH, Jagannathan JP, O'Regan K, Kim KW, Shinagare AB, Krajewski KM, et al. Molecular targeted therapies in non-GIST soft tissue sarcomas: what the radiologist needs to know. Cancer Imaging. 2013; 13:197–211.5. Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. World Health Organization classification of tumours of soft tissue and bone: pathology and genetics of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press;2013.6. Fletcher CD. The evolving classification of soft tissue tumours -an update based on the new 2013 WHO classification. Histopathology. 2014; 64:2–11.7. Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014; 46:95–104.8. Helman LJ, Maki RG. Sarcomas of soft tissue. 5th ed. Philadelphia: Elsevier;2014.9. Quesada J, Amato R. The molecular biology of soft-tissue sarcomas and current trends in therapy. Sarcoma. 2012; 2012:849456.10. Matushansky I, Maki RG. Mechanisms of sarcomagenesis. Hematol Oncol Clin North Am. 2005; 19:427–449. v11. Norberg SM, Movva S. Role of genetic and molecular profiling in sarcomas. Curr Treat Options Oncol. 2015; 16:24.12. Baheti AD, Tirumani SH, Sewatkar R, Shinagare AB, Hornick JL, Ramaiya NH, et al. Imaging features of primary and metastatic extremity synovial sarcoma: a single institute experience of 78 patients. Br J Radiol. 2015; 88:20140608.13. Murphey MD, Senchak LT, Mambalam PK, Logie CI, Klassen-Fischer MK, Kransdorf MJ. From the radiologic pathology archives: ewing sarcoma family of tumors: radiologic-pathologic correlation. Radiographics. 2013; 33:803–831.14. Somarouthu BS, Shinagare AB, Rosenthal MH, Tirumani H, Hornick JL, Ramaiya NH, et al. Multimodality imaging features, metastatic pattern and clinical outcome in adult extraskeletal Ewing sarcoma: experience in 26 patients. Br J Radiol. 2014; 87:20140123.15. Helman LJ, Maki RG. Sarcomas of soft tissue. In : Niederhuber JE, Armitage JO, Doroshow JH, Kastan MB, Tepper JE, editors. Abeloff's Clinical Oncology. 5th ed. Philadelphia: Elsevier;2014. p. 1753–1791.16. Nishino M, Jagannathan JP, Krajewski KM, O'Regan K, Hatabu H, Shapiro G, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012; 198:737–745.17. What are the key statistics about soft tissue sarcomas? Accessed Mar 29, 2016. Web site. http://www.cancer.org/cancer/sarcoma-adultsofttissuecancer/detailedguide/sarcoma-adult-soft-tissue-cancer-key-statistics.18. Murphey MD, Arcara LK, Fanburg-Smith J. From the archives of the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics. 2005; 25:1371–1395.19. O'Regan KN, Jagannathan J, Krajewski K, Zukotynski K, Souza F, Wagner AJ, et al. Imaging of liposarcoma: classification, patterns of tumor recurrence, and response to treatment. AJR Am J Roentgenol. 2011; 197:W37–W43.20. Wortman JR, Tirumani SH, Jagannathan JP, Tirumani H, Shinagare AB, Hornick JL, et al. Primary extremity liposarcoma: MRI features, histopathology, and clinical outcomes. J Comput Assist Tomogr. 2016; 40:791–798.21. Tirumani SH, Wagner AJ, Tirumani H, Shinagare AB, Jagannathan JP, Hornick JL, et al. Is the nonlipomatous component of dedifferentiated liposarcoma always soft tissue on CT? Analysis of CT densities and correlation with rate of growth in 60 patients. Abdom Imaging. 2015; 40:1248–1254.22. Brisson M, Kashima T, Delaney D, Tirabosco R, Clarke A, Cro S, et al. MRI characteristics of lipoma and atypical lipomatous tumor/well-differentiated liposarcoma: retrospective comparison with histology and MDM2 gene amplification. Skeletal Radiol. 2013; 42:635–647.23. O'Donnell PW, Griffin AM, Eward WC, Sternheim A, White LM, Wunder JS, et al. Can experienced observers differentiate between lipoma and well-differentiated liposarcoma using only MRI? Sarcoma. 2013; 2013:982784.24. Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002; 224:99–104.25. Baheti AD, Tirumani SH, Rosenthal MH, Howard SA, Shinagare AB, Ramaiya NH, et al. Myxoid soft-tissue neoplasms: comprehensive update of the taxonomy and MRI features. AJR Am J Roentgenol. 2015; 204:374–385.26. Löwenthal D, Zeile M, Niederhagen M, Fehlberg S, Schnapauff D, Pink D, et al. Differentiation of myxoid liposarcoma by magnetic resonance imaging: a histopathologic correlation. Acta Radiol. 2014; 55:952–960.27. van Vliet M, Kliffen M, Krestin GP, van Dijke CF. Soft tissue sarcomas at a glance: clinical, histological, and MR imaging features of malignant extremity soft tissue tumors. Eur Radiol. 2009; 19:1499–1511.28. Seo SW, Kwon JW, Jang SW, Jang SP, Park YS. Feasibility of whole-body MRI for detecting metastatic myxoid liposarcoma: a case series. Orthopedics. 2011; 34:e748–e754.29. Keung EZ, Hornick JL, Bertagnolli MM, Baldini EH, Raut CP. Predictors of outcomes in patients with primary retroperitoneal dedifferentiated liposarcoma undergoing surgery. J Am Coll Surg. 2014; 218:206–217.30. Wortman JR, Tirumani SH, Tirumani H, Shinagare AB, Jagannathan JP, Hornick JL, et al. Neoadjuvant radiation in primary extremity liposarcoma: correlation of MRI features with histopathology. Eur Radiol. 2016; 26:1226–1234.31. Di Giandomenico S, Frapolli R, Bello E, Uboldi S, Licandro SA, Marchini S, et al. Mode of action of trabectedin in myxoid liposarcomas. Oncogene. 2014; 33:5201–5210.32. Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016; 34:786–793.33. Saponara M, Stacchiotti S, Gronchi A. The safety and efficacy of trabectedin for the treatment of liposarcoma or leiomyosarcoma. Expert Rev Anticancer Ther. 2016; 16:473–484.34. Tirumani SH, Wagner AJ, Tirumani H, Shinagare AB, Jagannathan JP, Morgan JA, et al. Radiologic signs of adipocytic maturation (AM) in dedifferentiated liposarcoma (ddLPS) patients treated with trabectedin (T): correlation with disease control [abstract]. J Clin Oncol. 2014; 32:15 Suppl. Abstract no.10561.35. Wang WL, Katz D, Araujo DM, Ravi V, Ludwig JA, Trent JC, et al. Extensive adipocytic maturation can be seen in myxoid liposarcomas treated with neoadjuvant doxorubicin and ifosfamide and pre-operative radiation therapy. Clin Sarcoma Res. 2012; 2:25.36. Engström K, Bergh P, Cederlund CG, Hultborn R, Willen H, Aman P, et al. Irradiation of myxoid/round cell liposarcoma induces volume reduction and lipoma-like morphology. Acta Oncol. 2007; 46:838–845.37. Dickson MA, Tap WD, Keohan ML, D'Angelo SP, Gounder MM, Antonescu CR, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013; 31:2024–2028.38. Ray-Coquard I, Blay JY, Italiano A, Le Cesne A, Penel N, Zhi J, et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. Lancet Oncol. 2012; 13:1133–1140.39. O'Sullivan PJ, Harris AC, Munk PL. Radiological imaging features of non-uterine leiomyosarcoma. Br J Radiol. 2008; 81:73–81.40. Rosenberg AE. WHO classification of soft tissue and bone, fourth edition: summary and commentary. Curr Opin Oncol. 2013; 25:571–573.41. Gordon RW, Tirumani SH, Kurra V, Shinagare AB, Jagannathan JP, Hornick JL, et al. MRI, MDCT features, and clinical outcome of extremity leiomyosarcomas: experience in 47 patients. Skeletal Radiol. 2014; 43:615–622.42. Tirumani SH, Ojili V, Shanbhogue AK, Fasih N, Ryan JG, Reinhold C. Current concepts in the imaging of uterine sarcoma. Abdom Imaging. 2013; 38:397–411.43. Shah SH, Jagannathan JP, Krajewski K, O'Regan KN, George S, Ramaiya NH. Uterine sarcomas: then and now. AJR Am J Roentgenol. 2012; 199:213–223.44. D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010; 116:131–139.45. Tanaka YO, Nishida M, Tsunoda H, Okamoto Y, Yoshikawa H. Smooth muscle tumors of uncertain malignant potential and leiomyosarcomas of the uterus: MR findings. J Magn Reson Imaging. 2004; 20:998–1007.46. Schwartz LB, Zawin M, Carcangiu ML, Lange R, McCarthy S. Does pelvic magnetic resonance imaging differentiate among the histologic subtypes of uterine leiomyomata? Fertil Steril. 1998; 70:580–587.47. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002; 12:354–361.48. Sato K, Yuasa N, Fujita M, Fukushima Y. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am J Obstet Gynecol. 2014; 210:368.e1–368.e8.49. Amant F, Coosemans A, Debiec-Rychter M, Timmerman D, Vergote I. Clinical management of uterine sarcomas. Lancet Oncol. 2009; 10:1188–1198.50. Guntupalli SR, Ramirez PT, Anderson ML, Milam MR, Bodurka DC, Malpica A. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol. 2009; 113:324–326.51. Cooley CL, Jagannathan JP, Kurra V, Tirumani SH, Saboo SS, Ramaiya NH, et al. Imaging features and metastatic pattern of non-IVC retroperitoneal leiomyosarcomas: are they different from IVC leiomyosarcomas? J Comput Assist Tomogr. 2014; 38:687–692.52. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH Jr, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics. 2011; 31:949–976.53. Webb EM, Wang ZJ, Westphalen AC, Nakakura EK, Coakley FV, Yeh BM. Can CT features differentiate between inferior vena cava leiomyosarcomas and primary retroperitoneal masses? AJR Am J Roentgenol. 2013; 200:205–209.54. Tirumani SH, Deaver P, Shinagare AB, Tirumani H, Hornick JL, George S, et al. Metastatic pattern of uterine leiomyosarcoma: retrospective analysis of the predictors and outcome in 113 patients. J Gynecol Oncol. 2014; 25:306–312.55. D'Incalci M, Galmarini CM. A review of trabectedin (ET-743): a unique mechanism of action. Mol Cancer Ther. 2010; 9:2157–2163.56. van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012; 379:1879–1886.57. Rajendra R, Jones RL, Pollack SM. Targeted treatment for advanced soft tissue sarcoma: profile of pazopanib. Onco Targets Ther. 2013; 6:217–222.58. Penel N, Le Cesne A, Bui BN, Perol D, Brain EG, Ray-Coquard I, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol. 2011; 22:452–457.59. Chugh R, Wathen JK, Patel SR, Maki RG, Meyers PA, Schuetze SM, et al. Efficacy of imatinib in aggressive fibromatosis: Results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial. Clin Cancer Res. 2010; 16:4884–4891.60. Torreggiani WC, Al-Ismail K, Munk PL, Nicolaou S, O'Connell JX, Knowling MA. Dermatofibrosarcoma protuberans: MR imaging features. AJR Am J Roentgenol. 2002; 178:989–993.61. Kim GK. Status report on the management of dermatofibrosarcoma protuberans: is there a viable role for the use of imatinib mesylate? In which cases may it be therapeutically helpful and in which cases not? J Clin Aesthet Dermatol. 2011; 4:17–26.62. Rutkowski P, Dębiec-Rychter M, Nowecki Z, Michej W, Symonides M, Ptaszynski K, et al. Treatment of advanced dermatofibrosarcoma protuberans with imatinib mesylate with or without surgical resection. J Eur Acad Dermatol Venereol. 2011; 25:264–270.63. Labropoulos SV, Razis ED. Imatinib in the treatment of dermatofibrosarcoma protuberans. Biologics. 2007; 1:347–353.64. Demetri GD, Antonia S, Benjamin RS, Bui MM, Casper ES, Conrad EU 3rd, et al. Soft tissue sarcoma. J Natl Compr Canc Netw. 2010; 8:630–674.65. Imakiire N, Fujino T, Morii T, Honya K, Mochizuki K, Satomi K, et al. Malignant pigmented villonodular synovitis in the knee -report of a case with rapid clinical progression. Open Orthop J. 2011; 5:13–16.66. Snoots WM, Watkins D, Dockery D, Mennel R, Cheek BS. Pigmented villonodular synovitis responsive to imatinib therapy. Proc (Bayl Univ Med Cent). 2011; 24:134–138.67. Cassier PA, Gelderblom H, Stacchiotti S, Thomas D, Maki RG, Kroep JR, et al. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer. 2012; 118:1649–1655.68. Temple HT. Pigmented villonodular synovitis therapy with MSCF-1 inhibitors. Curr Opin Oncol. 2012; 24:404–408.69. Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016; 67:11–28.70. Huang Z, Wu Y, Zhou X, Qian J, Zhu W, Shu Y, et al. Clinical efficacy of mTOR inhibitors in solid tumors: a systematic review. Future Oncol. 2015; 11:1687–1699.71. Tirumani SH, Shinagare AB, Hargreaves J, Jagannathan JP, Hornick JL, Wagner AJ, et al. Imaging features of primary and metastatic malignant perivascular epithelioid cell tumors. AJR Am J Roentgenol. 2014; 202:252–258.72. Dickson MA, Schwartz GK, Antonescu CR, Kwiatkowski DJ, Malinowska IA. Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: clinical and molecular correlates. Int J Cancer. 2013; 132:1711–1717.73. Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010; 28:835–840.74. Lovly CM, Heuckmann JM, de Stanchina E, Chen H, Thomas RK, Liang C, et al. Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res. 2011; 71:4920–4931.75. Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003; 23:719–729.76. Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory pseudotumor: the great mimicker. AJR Am J Roentgenol. 2012; 198:W217–W227.77. Butrynski JE, D'Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010; 363:1727–1733.78. Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014; 4:889–895.79. de Bono JS, Yap TA. Future directions in the evaluation of c-MET-driven malignancies. Ther Adv Med Oncol. 2011; 3:1 Suppl. S51–S60.80. Giubellino A, Linehan WM, Bottaro DP. Targeting the Met signaling pathway in renal cancer. Expert Rev Anticancer Ther. 2009; 9:785–793.81. Sood S, Baheti AD, Shinagare AB, Jagannathan JP, Hornick JL, Ramaiya NH, et al. Imaging features of primary and metastatic alveolar soft part sarcoma: single institute experience in 25 patients. Br J Radiol. 2014; 87:20130719.82. Davis IJ, McFadden AW, Zhang Y, Coxon A, Burgess TL, Wagner AJ, et al. Identification of the receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as therapeutic targets in clear cell sarcoma. Cancer Res. 2010; 70:639–645.83. Wagner AJ, Goldberg JM, Dubois SG, Choy E, Rosen L, Pappo A, et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: results of a multicenter phase 2 trial. Cancer. 2012; 118:5894–5902.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gastrointestinal Stromal Tumors: Case Report, Aeromedical Assessment of Therapy
- A Case of Low-Grade Endometrial Stromal Sarcoma of the Uterus (So-Called ""Endolymphatic Stromal Myosis"")
- Hypertensive crisis during wide excision of gastrointestinal stromal cell tumor (GIST): Undiagnosed paraganglioma: A case report
- Primary Extragastrointestinal Stromal Tumor of Retroperitoneum: Poor Response to Tyrosine Kinase Inhibitor
- A Case of Massive Bleeding from Jejunal Stromal Tumor Diagnosed by Intraoperative Enteroscopy: A Case of Jejunal Stromal Tumor Bleeding