Korean J Radiol.
2017 Feb;18(1):54-70. 10.3348/kjr.2017.18.1.54.
Diffuse Large B-Cell Lymphoma in the Era of Precision Oncology: How Imaging Is Helpful
- Affiliations
-
- 1Department of Imaging, Dana Farber Cancer Institute, Harvard Medical School, Boston, MA 02215, USA. akeraliya@partners.org
- 2Department of Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115, USA.
- 3Department of Nuclear Medicine and PET/CT, Jaslok Hospital and Research Centre, Mumbai 400026, India.
- KMID: 2468122
- DOI: http://doi.org/10.3348/kjr.2017.18.1.54
Abstract
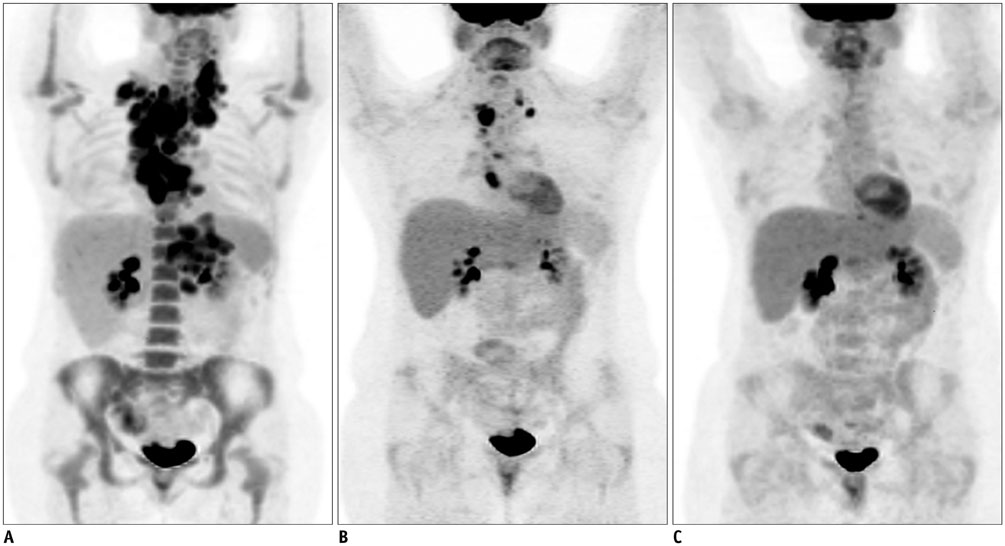
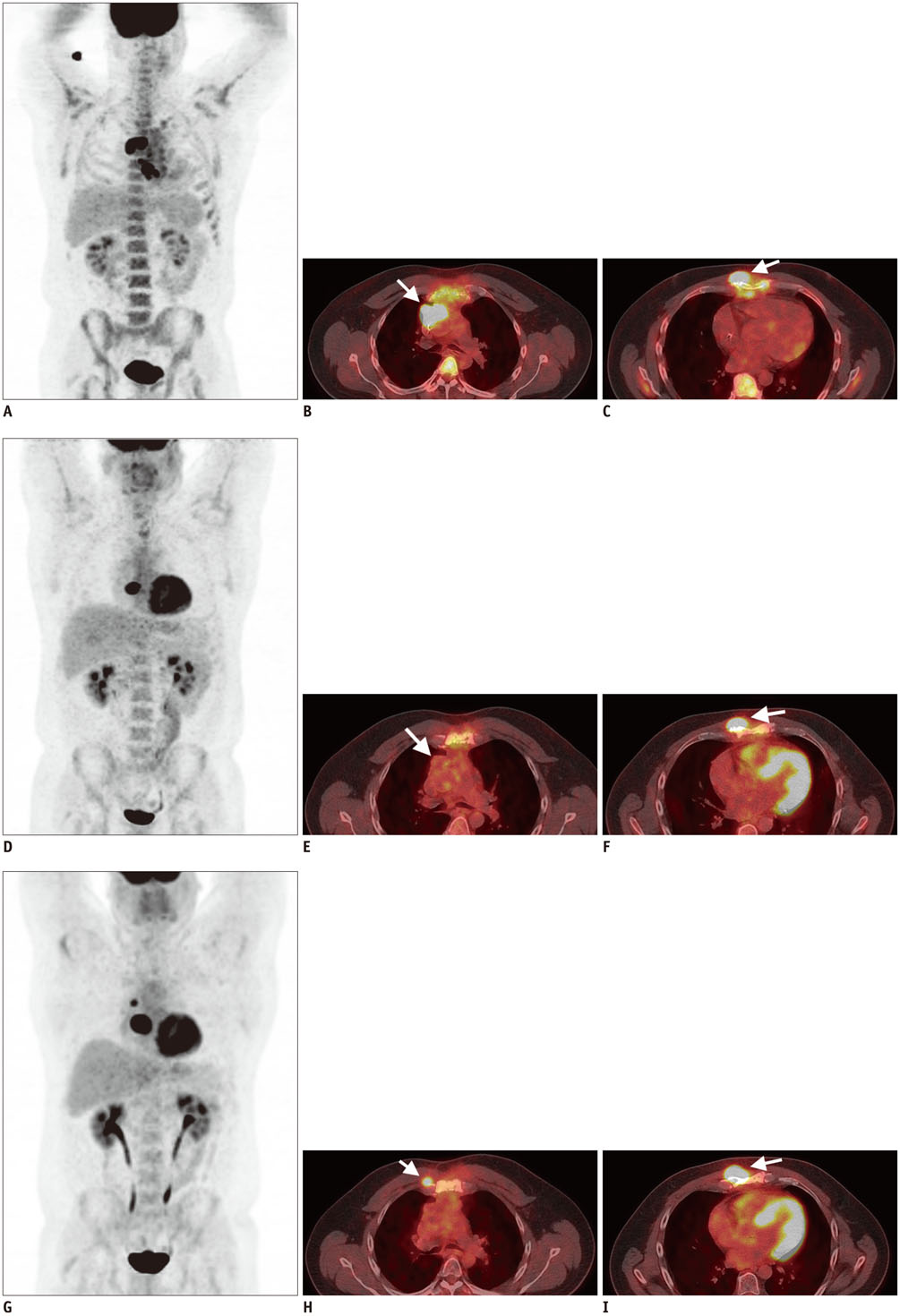
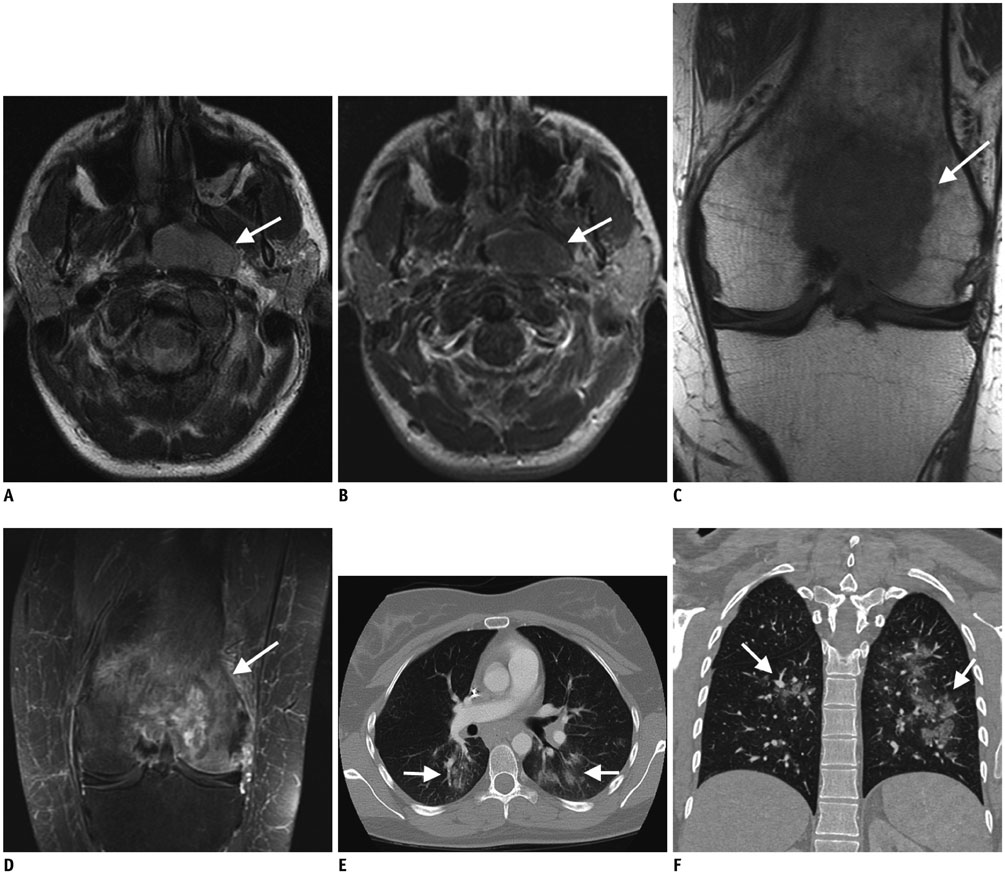
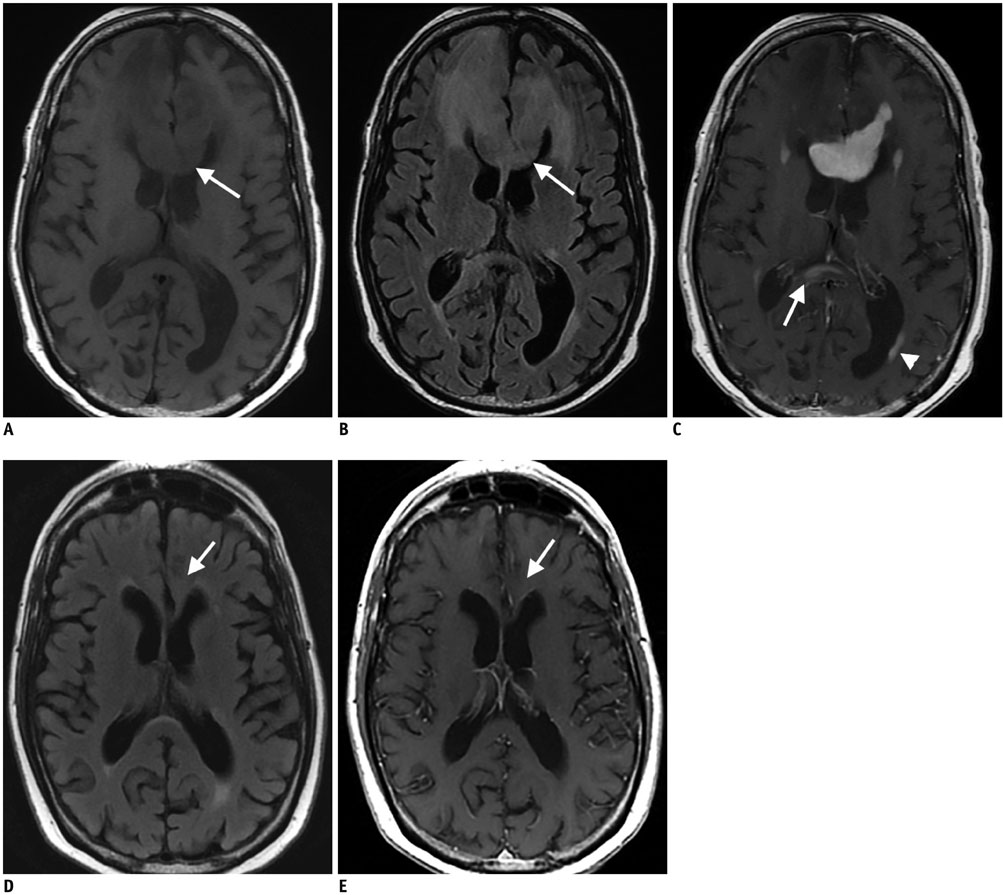
- Diffuse large B cell lymphoma (DLBCL) is the most common histological subtype of Non-Hodgkin's lymphoma. As treatments continues to evolve, so do imaging strategies, and positron emission tomography (PET) has emerged as the most important imaging tool to guide oncologists in the diagnosis, staging, response assessment, relapse/recurrence detection,and therapeutic decision making of DLBCL. Other imaging modalities including magnetic resonance imaging (MRI), computed tomography (CT), ultrasound, and conventional radiography are also used in the evaluation of lymphoma. MRI is useful for nervous system and musculoskeletal system involvement and is emerging as a radiation free alternative to PET/CT. This article provides a comprehensive review of both the functional and morphological imaging modalities, available in the management of DLBCL.
MeSH Terms
-
Female
Humans
Lymphoma, Large B-Cell, Diffuse/*diagnostic imaging/pathology/therapy
Magnetic Resonance Imaging/methods
Male
Multimodal Imaging/methods
Neoplasm Recurrence, Local
Positron Emission Tomography Computed Tomography/methods
Positron-Emission Tomography/methods
Precision Medicine/methods
Tomography, X-Ray Computed/methods
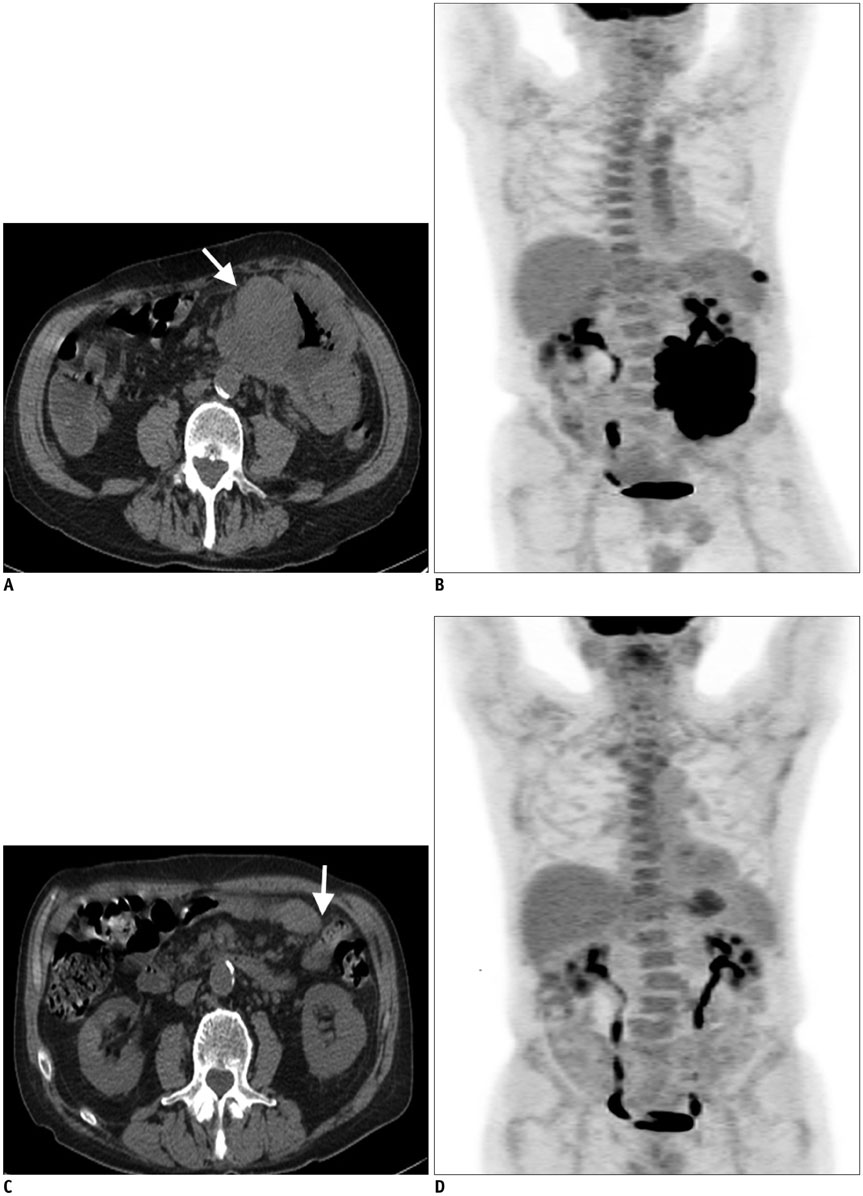
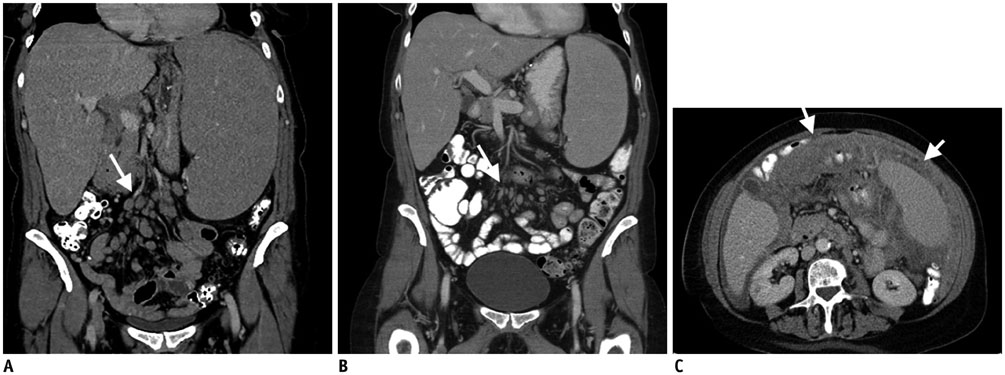
Figure
Cited by 1 articles
-
Diagnostic Accuracy and Prognostic Relevance of Immunoglobulin Heavy Chain Rearrangement and 18F-FDG-PET/CT Compared With Unilateral Bone Marrow Trephination for Detecting Bone Marrow Involvement in Patients With Diffuse Large B-Cell Lymphoma
Mihee Kim, Seo-Yeon Ahn, Jae-Sook Ahn, Ga-Young Song, Sung-Hoon Jung, Je-Jung Lee, Hyeoung-Joon Kim, Jun Hyung Lee, Myung-Geun Shin, Sang Yun Song, Deok-Hwan Yang
J Korean Med Sci. 2021;37(1):e2. doi: 10.3346/jkms.2022.37.e2.
Reference
-
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30.2. Møller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation--a population-based study of 1575 cases. Br J Haematol. 2004; 124:151–159.3. Shenoy PJ, Malik N, Nooka A, Sinha R, Ward KC, Brawley OW, et al. Racial differences in the presentation and outcomes of diffuse large B-cell lymphoma in the United States. Cancer. 2011; 117:2530–2540.4. Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008; 105:13520–13525.5. Iqbal J, Sanger WG, Horsman DE, Rosenwald A, Pickering DL, Dave B, et al. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol. 2004; 165:159–166.6. Basso K, Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol. 2010; 105:193–210.7. Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009; 114:3533–3537.8. Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001; 194:1861–1874.9. Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008; 319:1676–1679.10. Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010; 116:3268–3277.11. Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003; 198:851–862.12. Cazals-Hatem D, Lepage E, Brice P, Ferrant A, d'Agay MF, Baumelou E, et al. Primary mediastinal large B-cell lymphoma. A clinicopathologic study of 141 cases compared with 916 nonmediastinal large B-cell lymphomas, a GELA (“Groupe d'Etude des Lymphomes de l’Adulte“) study. Am J Surg Pathol. 1996; 20:877–888.13. Dunleavy K, Grant C, Eberle FC, Pittaluga S, Jaffe ES, Wilson WH. Gray zone lymphoma: better treated like Hodgkin lymphoma or mediastinal large B-cell lymphoma? Curr Hematol Malig Rep. 2012; 7:241–247.14. Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma EJ, et al. Double-hit B-cell lymphomas. Blood. 2011; 117:2319–2331.15. Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993; 328:1002–1006.16. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346:235–242.17. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010; 116:2040–2045.18. Batlevi CL, Matsuki E, Brentjens RJ, Younes A. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol. 2016; 13:25–40.19. Viardot A, Goebeler ME, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016; 127:1410–1416.20. Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015; 33:540–549.21. Sanford M. Blinatumomab: first global approval. Drugs. 2015; 75:321–327.22. Topp MS, Gökbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015; 16:57–66.23. Buie LW, Pecoraro JJ, Horvat TZ, Daley RJ. Blinatumomab: a first-in-class bispecific T-cell engager for precursor B-cell acute lymphoblastic leukemia. Ann Pharmacother. 2015; 49:1057–1067.24. Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016; 34:1104–1111.25. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–264.26. Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011; 364:2517–2526.27. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015; 372:311–319.28. Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013; 31:4199–4206.29. Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011; 2011:498–505.30. Raut LS, Chakrabarti PP. Management of relapsed-refractory diffuse large B cell lymphoma. South Asian J Cancer. 2014; 3:66–70.31. Ahuja AT, Ying M, Ho SY, Antonio G, Lee YP, King AD, et al. Ultrasound of malignant cervical lymph nodes. Cancer Imaging. 2008; 8:48–56.32. Ahuja AT, Ying M, Yuen HY, Metreweli C. ‘Pseudocystic’ appearance of non-Hodgkin's lymphomatous nodes: an infrequent finding with high-resolution transducers. Clin Radiol. 2001; 56:111–115.33. McInnes MD, Kielar AZ, Macdonald DB. Percutaneous image-guided biopsy of the spleen: systematic review and meta-analysis of the complication rate and diagnostic accuracy. Radiology. 2011; 260:699–708.34. Burke C, Thomas R, Inglis C, Baldwin A, Ramesar K, Grace R, et al. Ultrasound-guided core biopsy in the diagnosis of lymphoma of the head and neck. A 9 year experience. Br J Radiol. 2011; 84:727–732.35. Larcos G, Farlow DC, Antico VF, Gruenewald SM, Boyages J. The role of high dose 67-gallium scintigraphy in staging untreated patients with lymphoma. Aust N Z J Med. 1994; 24:5–8.36. Israel O, Front D, Epelbaum R, Ben-Haim S, Jerushalmi J, Kleinhaus U, et al. Residual mass and negative gallium scintigraphy in treated lymphoma. J Nucl Med. 1990; 31:365–368.37. Front D, Bar-Shalom R, Epelbaum R, Haim N, Ben-Arush MW, Ben-Shahar M, et al. Early detection of lymphoma recurrence with gallium-67 scintigraphy. J Nucl Med. 1993; 34:2101–2104.38. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–3068.39. NCCN. Non-Hodgkin's Lymphomas. Washington, DC: NCCN;2016.40. Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014; 123:837–842.41. Cheson BD. Staging and response assessment in lymphomas: the new Lugano classification. Chin Clin Oncol. 2015; 4:5.42. Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011; 29:1844–1854.43. Vriens D, Visser EP, de Geus-Oei LF, Oyen WJ. Methodological considerations in quantification of oncological FDG PET studies. Eur J Nucl Med Mol Imaging. 2010; 37:1408–1425.44. Kostakoglu L, Cheson BD. State-of-the-art research on “Lymphomas: role of molecular imaging for staging, prognostic evaluation, and treatment response”. Front Oncol. 2013; 3:212.45. Berthet L, Cochet A, Kanoun S, Berriolo-Riedinger A, Humbert O, Toubeau M, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med. 2013; 54:1244–1250.46. Adams HJ, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RA, de Klerk JM, et al. Bone marrow 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography cannot replace bone marrow biopsy in diffuse large B-cell lymphoma. Am J Hematol. 2014; 89:726–731.47. Cerci JJ, Györke T, Fanti S, Paez D, Meneghetti JC, Redondo F, et al. Combined PET and biopsy evidence of marrow involvement improves prognostic prediction in diffuse large B-cell lymphoma. J Nucl Med. 2014; 55:1591–1597.48. Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007; 25:571–578.49. Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the first international workshop on interim-PET-scan in lymphoma. Leuk Lymphoma. 2009; 50:1257–1260.50. Itti E, Meignan M, Berriolo-Riedinger A, Biggi A, Cashen AF, Véra P, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging. 2013; 40:1312–1320.51. Carr R, Fanti S, Paez D, Cerci J, Györke T, Redondo F, et al. Prospective international cohort study demonstrates inability of interim PET to predict treatment failure in diffuse large B-cell lymphoma. J Nucl Med. 2014; 55:1936–1944.52. Dührsen U, Hüttmann A, Jöckel KH, Müller S. Positron emission tomography guided therapy of aggressive non-Hodgkin lymphomas--the PETAL trial. Leuk Lymphoma. 2009; 50:1757–1760.53. van der Kolk LE, Grillo-López AJ, Baars JW, Hack CE, van Oers MH. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001; 115:807–811.54. Hadjinicolaou AV, Nisar MK, Parfrey H, Chilvers ER, Ostör AJ. Non-infectious pulmonary toxicity of rituximab: a systematic review. Rheumatology (Oxford). 2012; 51:653–662.55. Adams HJ, Nievelstein RA, Kwee TC. Prognostic value of complete remission status at end-of-treatment FDG-PET in R-CHOP-treated diffuse large B-cell lymphoma: systematic review and meta-analysis. Br J Haematol. 2015; 170:185–191.56. Coughlan M, Elstrom R. The use of FDG-PET in diffuse large B cell lymphoma (DLBCL): predicting outcome following first line therapy. Cancer Imaging. 2014; 14:34.57. Cremerius U, Fabry U, Wildberger JE, Zimny M, Reinartz P, Nowak B, et al. Pre-transplant positron emission tomography (PET) using fluorine-18-fluoro-deoxyglucose (FDG) predicts outcome in patients treated with high-dose chemotherapy and autologous stem cell transplantation for non-Hodgkin's lymphoma. Bone Marrow Transplant. 2002; 30:103–111.58. Sauter CS, Matasar MJ, Meikle J, Schoder H, Ulaner GA, Migliacci JC, et al. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood. 2015; 125:2579–2581.59. Brastianos PK, Batchelor TT. Primary central nervous system lymphoma: overview of current treatment strategies. Hematol Oncol Clin North Am. 2012; 26:897–916.60. Korfel A, Schlegel U. Diagnosis and treatment of primary CNS lymphoma. Nat Rev Neurol. 2013; 9:317–327.61. Mohile NA, Abrey LE. Primary central nervous system lymphoma. Neurol Clin. 2007; 25:1193–1207. xi62. Grisariu S, Avni B, Batchelor TT, van den Bent MJ, Bokstein F, Schiff D, et al. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2010; 115:5005–5011.63. Bollen EL, Brouwer RE, Hamers S, Hermans J, Kluin M, Sankatsing SU, et al. Central nervous system relapse in non-Hodgkin lymphoma. A single-center study of 532 patients. Arch Neurol. 1997; 54:854–859.64. Mohile NA, Deangelis LM, Abrey LE. The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro Oncol. 2008; 10:223–228.65. Mayerhoefer ME, Karanikas G, Kletter K, Prosch H, Kiesewetter B, Skrabs C, et al. Evaluation of diffusion-weighted MRI for pretherapeutic assessment and staging of lymphoma: results of a prospective study in 140 patients. Clin Cancer Res. 2014; 20:2984–2993.66. Littooij AS, Kwee TC, Barber I, Granata C, Vermoolen MA, Enríquez G, et al. Whole-body MRI for initial staging of paediatric lymphoma: prospective comparison to an FDG-PET/CT-based reference standard. Eur Radiol. 2014; 24:1153–1165.67. Heacock L, Weissbrot J, Raad R, Campbell N, Friedman KP, Ponzo F, et al. PET/MRI for the evaluation of patients with lymphoma: initial observations. AJR Am J Roentgenol. 2015; 204:842–848.68. Herrmann K, Queiroz M, Huellner MW, de Galiza Barbosa F, Buck A, Schaefer N, et al. Diagnostic performance of FDG-PET/MRI and WB-DW-MRI in the evaluation of lymphoma: a prospective comparison to standard FDG-PET/CT. BMC Cancer. 2015; 15:1002.69. Riedel DJ, Rositch AF, Redfield RR, Blattner WA. HIV-associated lymphoma sub-type distribution, immunophenotypes and survival in an urban clinic population. Leuk Lymphoma. 2016; 57:306–312.70. Agarwal PA, Menon S, Smruti BK, Singhal BS. Primary central nervous system lymphoma: a profile of 26 cases from Western India. Neurol India. 2009; 57:756–763.71. Bayraktar S, Bayraktar UD, Ramos JC, Stefanovic A, Lossos IS. Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol. 2011; 101:257–265.72. Johnson BA, Fram EK, Johnson PC, Jacobowitz R. The variable MR appearance of primary lymphoma of the central nervous system: comparison with histopathologic features. AJNR Am J Neuroradiol. 1997; 18:563–572.73. Hare SS, Souza CA, Bain G, Seely JM, Frcpc , Gomes MM, et al. The radiological spectrum of pulmonary lymphoproliferative disease. Br J Radiol. 2012; 85:848–864.74. Anis M, Irshad A. Imaging of abdominal lymphoma. Radiol Clin North Am. 2008; 46:265–285. viii–ix.75. Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998; 4:1334–1336.76. Leng K. Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. 3'-Deoxy-3'-[18F]fluorothymidine. Accessed January 15, 2015. http://www.ncbi.nlm.nih.gov/books/NBK23373/.77. Sherley JL, Kelly TJ. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988; 263:8350–8358.78. Tian J, Yang X, Yu L, Chen P, Xin J, Ma L, et al. A multicenter clinical trial on the diagnostic value of dual-tracer PET/CT in pulmonary lesions using 3'-deoxy-3'-18F-fluorothymidine and 18F-FDG. J Nucl Med. 2008; 49:186–194.79. Zhao S, Kuge Y, Kohanawa M, Takahashi T, Zhao Y, Yi M, et al. Usefulness of 11C-methionine for differentiating tumors from granulomas in experimental rat models: a comparison with 18F-FDG and 18F-FLT. J Nucl Med. 2008; 49:135–141.80. Troost EG, Vogel WV, Merkx MA, Slootweg PJ, Marres HA, Peeters WJ, et al. 18F-FLT PET does not discriminate between reactive and metastatic lymph nodes in primary head and neck cancer patients. J Nucl Med. 2007; 48:726–735.81. Tadmor T, Shvidel L, Bairey O, Goldschmidt N, Ruchlemer R, Fineman R, et al. Richter's transformation to diffuse large B-cell lymphoma: a retrospective study reporting clinical data, outcome, and the benefit of adding rituximab to chemotherapy, from the Israeli CLL Study Group. Am J Hematol. 2014; 89:E218–E222.82. Giardino AA, O'Regan K, Jagannathan JP, Elco C, Ramaiya N, Lacasce A. Richter's transformation of chronic lymphocytic leukemia. J Clin Oncol. 2011; 29:e274–e276.83. Bruzzi JF, Macapinlac H, Tsimberidou AM, Truong MT, Keating MJ, Marom EM, et al. Detection of Richter's transformation of chronic lymphocytic leukemia by PET/CT. J Nucl Med. 2006; 47:1267–1273.84. Falchi L, Keating MJ, Marom EM, Truong MT, Schlette EJ, Sargent RL, et al. Correlation between FDG/PET, histology, characteristics, and survival in 332 patients with chronic lymphoid leukemia. Blood. 2014; 123:2783–2790.85. Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009; 523–531.86. Zuckerman D, Seliem R, Hochberg E. Intravascular lymphoma: the oncologist's “great imitator”. Oncologist. 2006; 11:496–502.87. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. 4th ed. Lyon: IARC;2008.88. Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009; 10:895–902.89. Yamamoto A, Kikuchi Y, Homma K, O'uchi T, Furui S. Characteristics of intravascular large B-cell lymphoma on cerebral MR imaging. AJNR Am J Neuroradiol. 2012; 33:292–296.90. Huntington SF, Svoboda J, Doshi JA. Cost-effectiveness analysis of routine surveillance imaging of patients with diffuse large B-cell lymphoma in first remission. J Clin Oncol. 2015; 33:1467–1474.91. Thompson CA, Ghesquieres H, Maurer MJ, Cerhan JR, Biron P, Ansell SM, et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol. 2014; 32:3506–3512.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffuse large B-cell lymphoma presenting with cholecystitis-like symptoms
- A Case of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma Occurring in Thyroid Gland
- Relapse of Ocular Lymphoma following Primary Testicular Diffuse Large B-cell Lymphoma
- MR Imaging Features of Primary Cutaneous Diffuse Large B-cell Lymphoma: A Case Report
- Primary Diffuse Large B Cell Lymphoma Developing at the Ileocolonic Anastomosis Site after Right Hemicolectomy for Adenocarcinoma: A Case Report