J Bacteriol Virol.
2019 Dec;49(4):203-211. 10.4167/jbv.2019.49.4.203.
Antiviral Activity of Gemcitabine Against Echovirus 30 Infection in Vitro
- Affiliations
-
- 1Department of Beauty Science, Kwangju Women's University, 165 Sanjeong-dong, Gwangsan-gu, Gwangju 62396, Korea.
- 2Laboratory of Microbiology and Immunology, College of Pharmacy, Kangwon National University, Chuncheon 24341, Korea.
- 3Department of Clinical Pathology, Daejeon Health Institute of Technology, Daejeon 34504, Korea. kayun@hit.ac.kr
- KMID: 2468026
- DOI: http://doi.org/10.4167/jbv.2019.49.4.203
Abstract
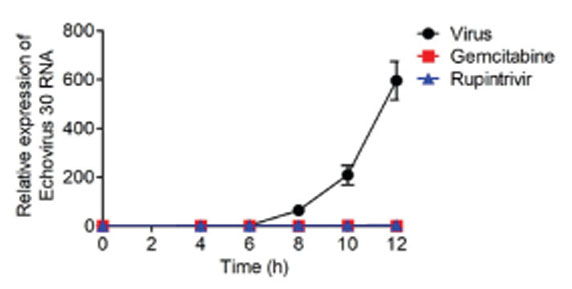
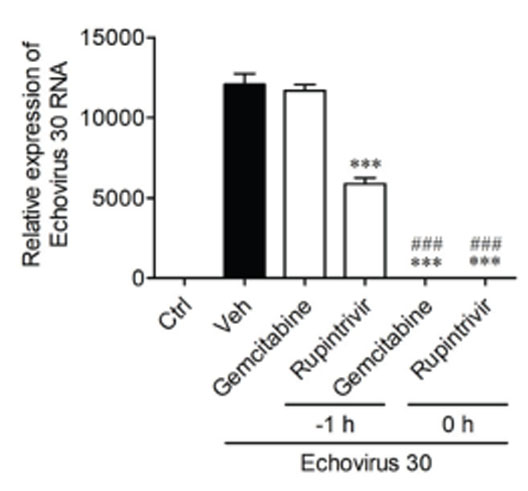
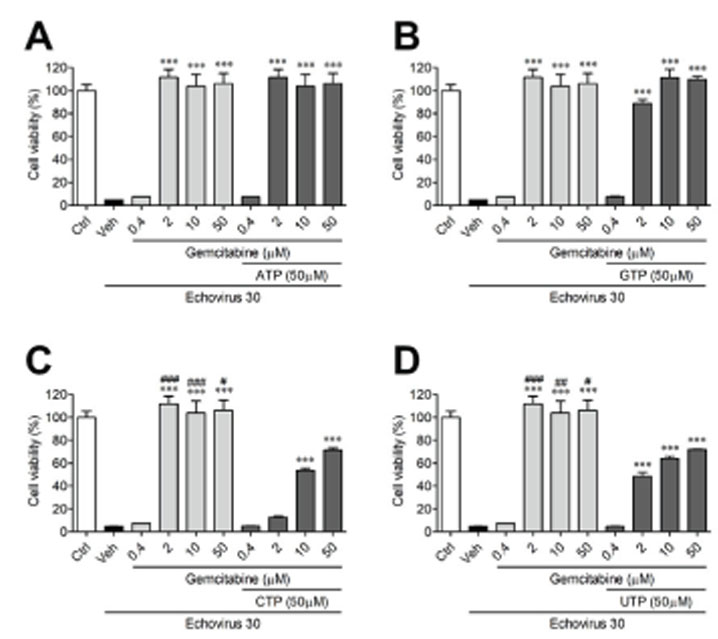
- Echovirus 30 is one of the major causes of meningitis in children and adults. The purpose of our current study was to investigate whether selected antiviral drugs could provide antiviral activity against echovirus 30. Using RD cells, we assessed the cytopathic effect of echovirus 30, including viral RNA levels as indicators of viral replication. The effects of gemcitabine were compared to rupintrivir, a well-known antiviral drug. To understand the activity gemcitabine exerts on the viral life cycle, time course and time-of-addition assays were implemented. The most effective compounds against echovirus 30 were gemcitabine and rupintrivir, as demonstrated by their concentration-dependent activity. Gemcitabine affects the early stages of echovirus 30 infection by disrupting viral replication. However, gemcitabine failed to directly inactivate echovirus 30 particles or impede viral uptake into the RD cells. Gemcitabine can be considered as a lead candidate in the development of echovirus 30 antiviral drugs, specifically in the early stages of echovirus 30 replication.
Keyword
MeSH Terms
Figure
Reference
-
1. Fieldhouse JK, Wang X, Mallinson KA, Tsao RW, Gray GC. A systematic review of evidence that enteroviruses may be zoonotic. Emerg Microbes Infect. 2018; 7:164.
Article2. Harvala H, Calvert J, Van Nguyen D, Clasper L, Gadsby N, Molyneaux P, et al. Comparison of diagnostic clinical samples and environmental sampling for enterovirus and parechovirus surveillance in Scotland, 2010 to 2012. Euro surveill. 2014; 19:20772.
Article3. Maguire HC, Atkinson P, Sharland M, Bendig J. Enterovirus infections in England and Wales: laboratory surveillance data: 1975 to 1994. Commun Dis Public Health. 1999; 2:122–125.4. Sharp J, Harrison CJ, Puckett K, Selvaraju SB, Penaranda S, Nix WA, et al. Characteristics of young infants in whom human parechovirus, enterovirus or neither were detected in cerebrospinal fluid during sepsis evaluations. Pediatr Infect Dis J. 2013; 32:213–216.
Article5. Peigue-Lafeuille H, Croquez N, Laurichesse H, Clavelou P, Aumaître O, Schmidt J, et al. Enterovirus meningitis in adults in 1999-2000 and evaluation of clinical management. J Med Virol. 2002; 67:47–53.
Article6. Amvrosieva TV, Titov LP, Mulders M, Hovi T, Dyakonova OV, Votyakov VI, et al. Viral water contamination as the cause of aseptic meningitis outbreak in Belarus. Cent Eur J Public Health. 2001; 9:154–157.7. Drebot MA, Nguan CY, Campbell JJ, Lee SH, Forward KR. Molecular epidemiology of enterovirus outbreaks in Canada during 1991-1992: identification of echovirus 30 and coxsackievirus B1 strains by amplicon sequencing. J Med Virol. 1994; 44:340–347.
Article8. Lévêque N, Jacques J, Renois F, Antona D, Abely M, Chomel JJ, et al. Phylogenetic analysis of Echovirus 30 isolated during the 2005 outbreak in France reveals existence of multiple lineages and suggests frequent recombination events. J Clin Virol. 2010; 48:137–141.
Article9. Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance--United States, 1970-2005. MMWR Surveill Summ. 2006; 55:1–20.10. Palacios G, Casas I, Cisterna D, Trallero G, Tenorio A, Freire C. Molecular epidemiology of echovirus 30: temporal circulation and prevalence of single lineages. J Virol. 2002; 76:4940–4949.
Article11. McWilliam Leitch EC, Bendig J, Cabrerizo M, Cardosa J, Hyypiä T, Ivanova OE, et al. Transmission networks and population turnover of echovirus 30. J Virol. 2009; 83:2109–2118.
Article12. Metcalf CJE, Lessler J. Opportunities and challenges in modeling emerging infectious diseases. Science. 2017; 357:149–152.
Article13. De Rycker M, Baragaña B, Duce SL, Gilbert IH. Challenges and recent progress in drug discovery for tropical diseases. Nature. 2018; 559:498–506.
Article14. Baker S, Thomson N, Weill FX, Holt KE. Genomic insights into the emergence and spread of antimicrobial-resistant bacterial pathogens. Science. 2018; 360:733–738.
Article15. Meylan S, Andrews IW, Collins JJ. Targeting Antibiotic Tolerance, Pathogen by Pathogen. Cell. 2018; 172:1228–1238.
Article16. Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol. 2011; 9:233–243.
Article17. Baig J, Shokouh-Amiri M, Chan J, Chowdhery R, Danthurthy S, Venepalli NK. The Spectrum of Pulmonary Toxicity in Pancreatic Cancer Patients Receiving Gemcitabine Combination Chemotherapy. Case Rep Oncol. 2019; 12:506–512.
Article18. Song J, Yeo SG, Hong EH, Lee BR, Kim JW, Kim J, et al. Antiviral Activity of Hederasaponin B from Hedera helix against Enterovirus 71 Subgenotypes C3 and C4a. Biomol Ther. 2014; 22:41–46.
Article19. Choi HJ, Kim JH, Lee CH, Ahn YJ, Song JH, Baek SH, et al. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009; 81:77–81.
Article20. Kwon BE, Song JH, Song HH, Kang JW, Hwang SN, Rhee KJ, et al. Antiviral Activity of Oroxylin A against Coxsackievirus B3 Alleviates Virus-Induced Acute Pancreatic Damage in Mice. PloS one. 2016; 11:e0155784.
Article21. Song JH, Kim SR, Heo EY, Lee JY, Kim DE, Cho S, et al. Antiviral activity of gemcitabine against human rhinovirus in vitro and in vivo. Antiviral Res. 2017; 145:6–13.
Article22. Heinemann V, Schulz L, Issels RD, Plunkett W. Gemcitabine: a modulator of intracellular nucleotide and deoxynucleotide metabolism. Semin Oncol. 1995; 22:11–18.23. Nyanguile O. Peptide Antiviral Strategies as an Alternative to Treat Lower Respiratory Viral Infections. Front Immunol. 2019; 10:1366.
Article24. Cornacchia MA, Coromilas AJ, Gallitano SM, Frank RC, Halasz CL. Subacute cutaneous lupus erythematosus induced by gemcitabine in 2 patients with pancreatic cancer. JAAD Case Rep. 2019; 5:596–601.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiviral Activity of Itraconazole against Echovirus 30 Infection In Vitro
- Echovirus-7 Infection in Children with Unusual Severe Manifestation: A Case Report
- Utility of 293 Cells in Culture of Enteroviruses
- Antiviral Activity of Lactobacillus spp. and Polysaccharide
- Sequencing and Molecular Characterization of the Genome of Echovirus 30 Isolated from a Korean Aseptic Meningitis Patient