Transl Clin Pharmacol.
2019 Dec;27(4):115-118. 10.12793/tcp.2019.27.4.115.
Korean clinical trials: its current status, future prospects, and enabling environment
- Affiliations
-
- 1Korea National Enterprise for Clinical Trials, KoNECT, Seoul 04143, Korea. deborah.chee@konect.or.kr
- KMID: 2467963
- DOI: http://doi.org/10.12793/tcp.2019.27.4.115
Abstract
- No abstract available.
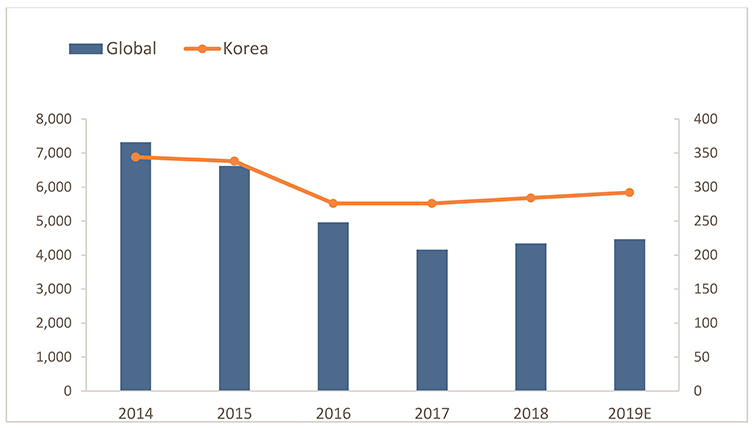
Figure
Reference
-
1. Accessed 1 November 2019. https://www.koreaclinicaltrials.org/kr/contents/datainfo_data_01_tab02/view.do.2. KoNECT annual analysis of ClinicalTrials.gov database. Internal data. 2019. 08.3. Accessed 1 November 2019. http://www.korea.kr/news/pressReleaseView.do?newsId=155943465.4. Accessed1 November 2019. https://www.mfds.go.kr/brd/m_99/view.do?seq=43284&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1.5. KoNECT annual analysis of MFDS clinical trial authorization data, Internal data.6. KoNECT survey on clinical trial activities of companies operating in Korea. Internal data. 2018.7. Clinical Trials Statistics. KoNECT;2018. p. 22–23.8. Contract research organizations global market opportunities and strategies to 2021, the business research company. 2018. 07. p. 30–31.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status and Future Prospect of Robotic Surgery in Korea
- The first step to the powers for clinical trials: a survey on the current and future Clinical Trial Management System
- Current aspects and prospects of glass ionomer cements for clinical dentistry
- Revascularization for Left Main and Multivessel Coronary Artery Disease: Current Status and Future Prospects after the EXCEL and NOBLE Trials
- A Study on Current Status of Clinical Trial Pharmacy in Domestic Clinical Trial Institution