J Clin Neurol.
2019 Oct;15(4):480-487. 10.3988/jcn.2019.15.4.480.
‘Sirim’ (Cold) Pain as a Common Symptom in Korean Patients with Clinically Suspected Small-Fiber Neuropathy
- Affiliations
-
- 1Department of Neurology, College of Medicine, Gyeongsang Institute of Health Science, Gyeongsang National University, Jinju, Korea.
- 2Department of Neurology, Gyeongsang National University Changwon Hospital, Changwon, Korea.
- 3Department of Neurology, Soonchunhyang University College of Medicine, Cheonan Hospital, Cheonan, Korea.
- 4Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. bjkim@skku.edu
- 5Department of Neurology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2467757
- DOI: http://doi.org/10.3988/jcn.2019.15.4.480
Abstract
- BACKGROUND AND PURPOSE
Diagnosing small-fiber neuropathy (SFN) is challenging because there is no gold-standard test and few diagnostic tests. This study investigated the clinical symptom profile and its associations with the results of quantitative sensory testing (QST) and the quantitative sudomotor axon reflex test (QSART) as well as the quality of life (QOL) in patients with clinically suspected SFN.
METHODS
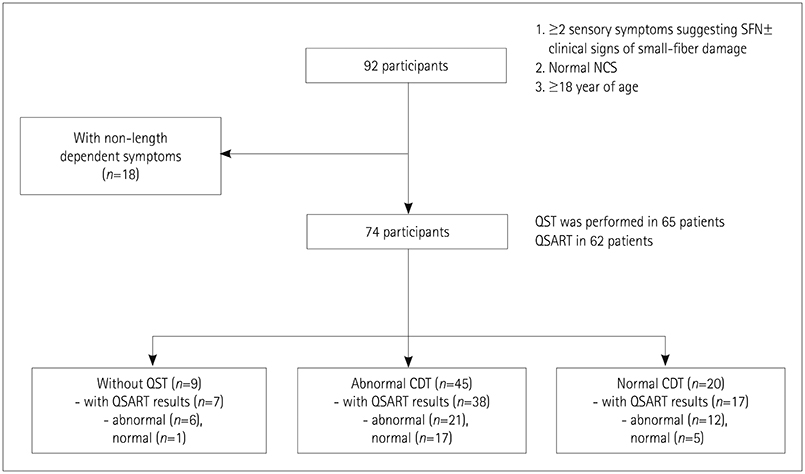
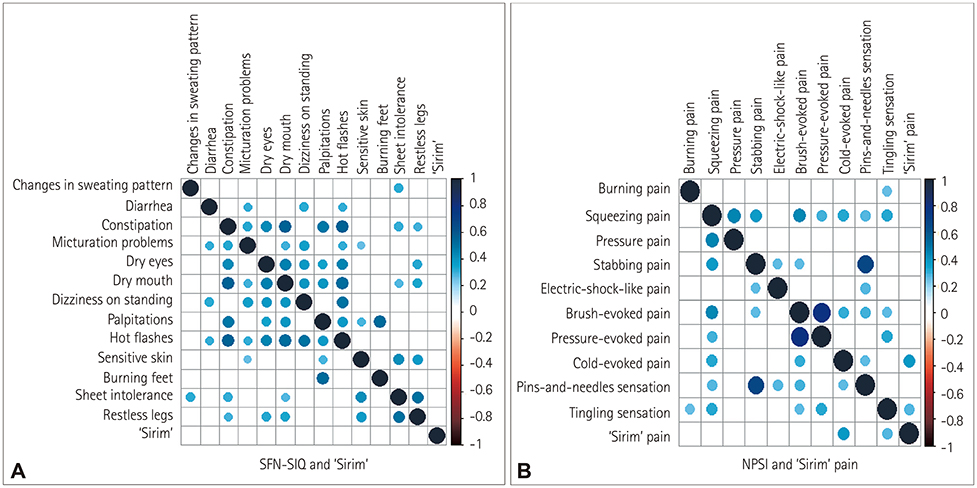
This study involved 63 patients with clinically suspected length-dependent SFN. Assessments were performed using QST, QSART, SFN Symptoms Inventory Questionnaire, Neuropathic Pain Symptom Inventory, "˜Sirim' frequency and "˜Sirim' (cold) pain severity, and 36-item Short-Form Health Survey. Multiple logistic and linear regression analyses were performed to predict risk factors for QST or QSART abnormalities and QOL, respectively.
RESULTS
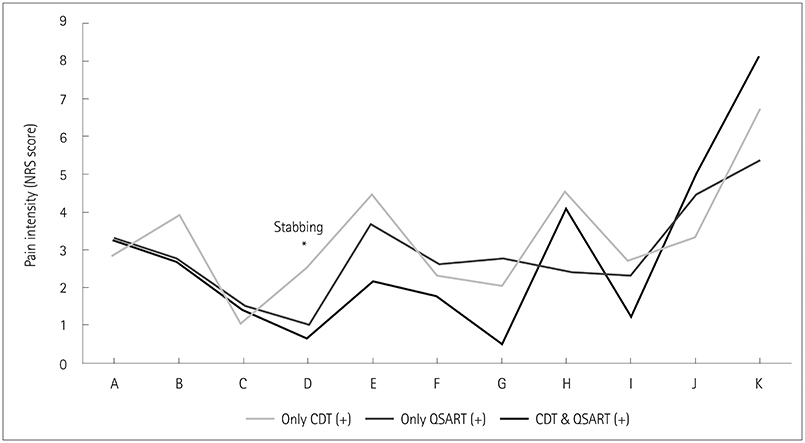
"˜Sirim' and "˜Sirim' pain was the most-common (84%) and the most-severe complaint (mean score of 6.3 on a numerical rating scale ranging from 0 to 10) in patients with clinically suspected SFN. The findings of QST [cold detection threshold (CDT)] and QSART were abnormal in 71% (n=45/57) and 62% (n=39/56) of the patients, respectively. An abnormal CDT was correlated with more-severe stabbing pain (odds ratio=2.23, 95% CI=1.02-4.87, p=0.045). Restless-leg symptoms (β=−7.077) and pressure-evoked pain (β=−5.034) were independent predictors of the physical aspects of QOL.
CONCLUSIONS
"˜Sirim' pain, similar to cold pain, should be considered a major neuropathic pain in SFN. Among pain characteristics, stabbing pain of a spontaneous paroxysmal nature may be more pronounced in the setting of dysfunctional Aδ fibers with functional autonomic C fibers.
Keyword
MeSH Terms
Figure
Reference
-
1. Khoshnoodi MA, Truelove S, Burakgazi A, Hoke A, Mammen AL, Polydefkis M. Longitudinal assessment of small fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol. 2016; 73:684–690.2. Terkelsen AJ, Karlsson P, Lauria G, Freeman R, Finnerup NB, Jensen TS. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol. 2017; 16:934–944.
Article3. De Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy-a large cohort study and review of the literature. Eur J Neurol. 2018; 25:348–355.
Article4. Johnson JM, Kellogg DL Jr. Local thermal control of the human cutaneous circulation. J Appl Physiol (1985). 2010; 109:1229–1238.
Article5. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010; 33:2285–2293.
Article6. Preston DC, Shapiro BE. Electromyography and neuromuscular disorders: clinical-electrophysiologic correlations. 3th ed. Philadelphia: Elsevier Saunders;2012.7. Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912–1925.
Article8. Cruccu G, Truini A. Tools for assessing neuropathic pain. PLoS Med. 2009; 6:e1000045.
Article9. Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in the nociceptive flexion reflex (NFR). Pain. 2008; 134:91–96.
Article10. Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001; 63:316–323.
Article11. Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med. 2005; 67:948–956.
Article12. Ochoa JL, Yarnitsky D. The triple cold syndrome. Cold hyperalgesia, cold hypoaesthesia and cold skin in peripheral nerve disease. Brain. 1994; 117:185–197.13. Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Front Biosci (Landmark Ed). 2010; 15:718–739.
Article14. Campero M, Baumann TK, Bostock H, Ochoa JL. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol. 2009; 587:5633–5652.
Article15. Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014; 13:924–935.
Article16. Yin K, Zimmermann K, Vetter I, Lewis RJ. Therapeutic opportunities for targeting cold pain pathways. Biochem Pharmacol. 2015; 93:125–140.
Article17. Moeller-Bertram T, Schilling JM, Bačkonja MM, Nemenov MI. Sensory small fiber function differentially assessed with diode laser (DL) quantitative sensory testing (QST) in painful neuropathy (PN). Pain Med. 2013; 14:417–421.
Article18. Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017; 158:261–272.
Article19. Krämer HH, Rolke R, Bickel A, Birklein F. Thermal thresholds predict painfulness of diabetic neuropathies. Diabetes Care. 2004; 27:2386–2391.
Article20. Ng Wing Tin S, Ciampi de Andrade D, Goujon C, Planté-Bordeneuve V, Créange A, Lefaucheur JP. Sensory correlates of pain in peripheral neuropathies. Clin Neurophysiol. 2014; 125:1048–1058.
Article21. Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN. Autonomic evaluation is independent of somatic evaluation for small fiber neuropathy. J Neurol Sci. 2014; 344:51–54.
Article22. Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN. Contribution of QSART to the diagnosis of small fiber neuropathy. Muscle Nerve. 2013; 48:883–888.
Article23. Bakkers M, Faber CG, Hoeijmakers JG, Lauria G, Merkies IS. Small fibers, large impact: quality of life in small-fiber neuropathy. Muscle Nerve. 2014; 49:329–336.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quantitative sudomotor axon reflex test (QSART) as a diagnostic tool of small fiber neuropathy
- Skin Biopsy: Emerging Method for Small Nerve Fiber Evaluation
- A Case of Autonomic Dysfunction and Painful Sensory Neuropathy in Sjogren's Syndrome
- Skin biopsy: an emerging method for small nerve fiber evaluation
- Peripheral Neuropathy and Pain Caused by Cancer Chemotherapy