Korean J Clin Neurophysiol.
2015 Dec;17(2):53-60. 10.14253/kjcn.2015.17.2.53.
Skin Biopsy: Emerging Method for Small Nerve Fiber Evaluation
- Affiliations
-
- 1Department of Neurology, Chungnam National University Hospital, Daejeon, Korea. seh337@daum.net
- KMID: 2403239
- DOI: http://doi.org/10.14253/kjcn.2015.17.2.53
Abstract
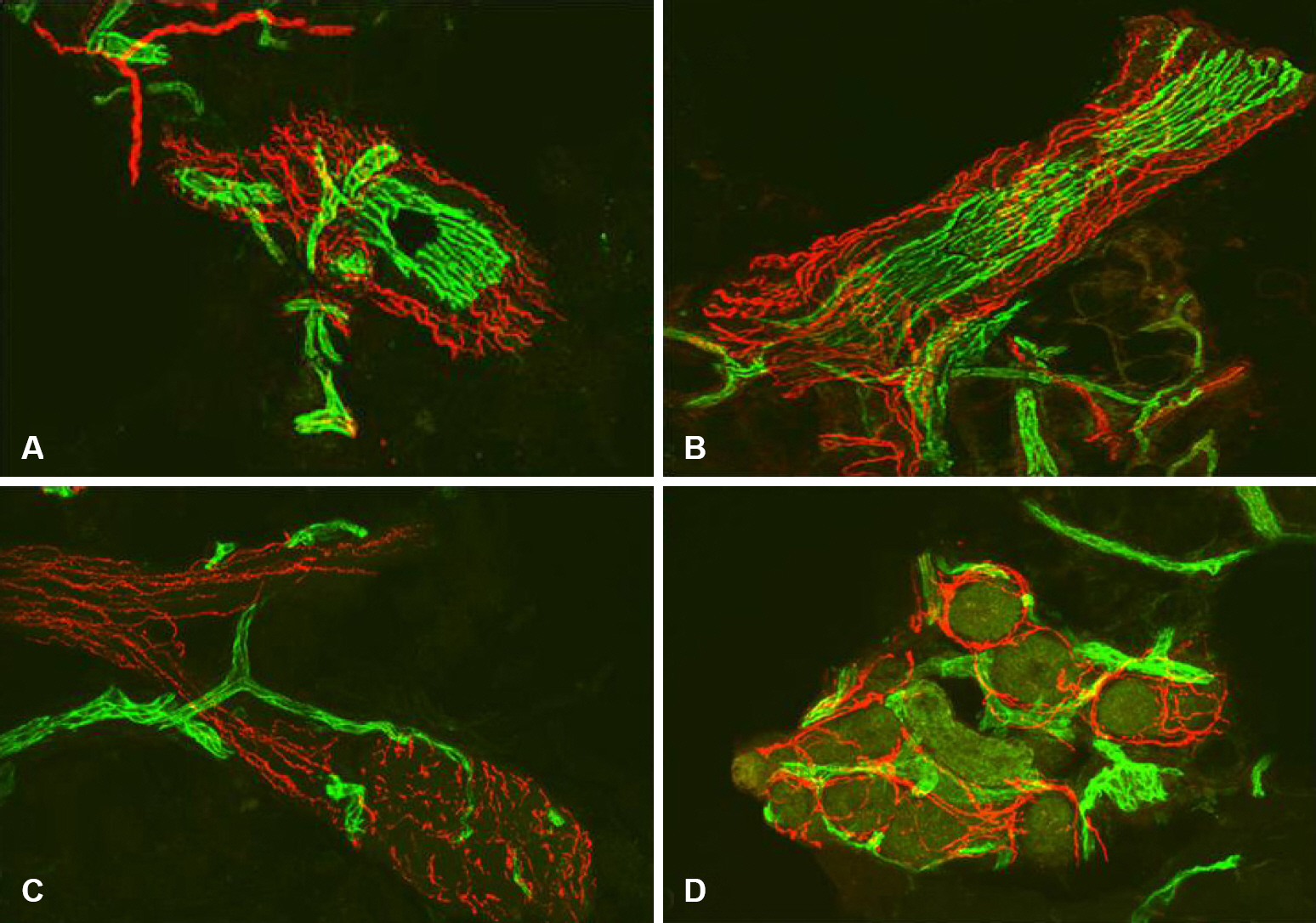
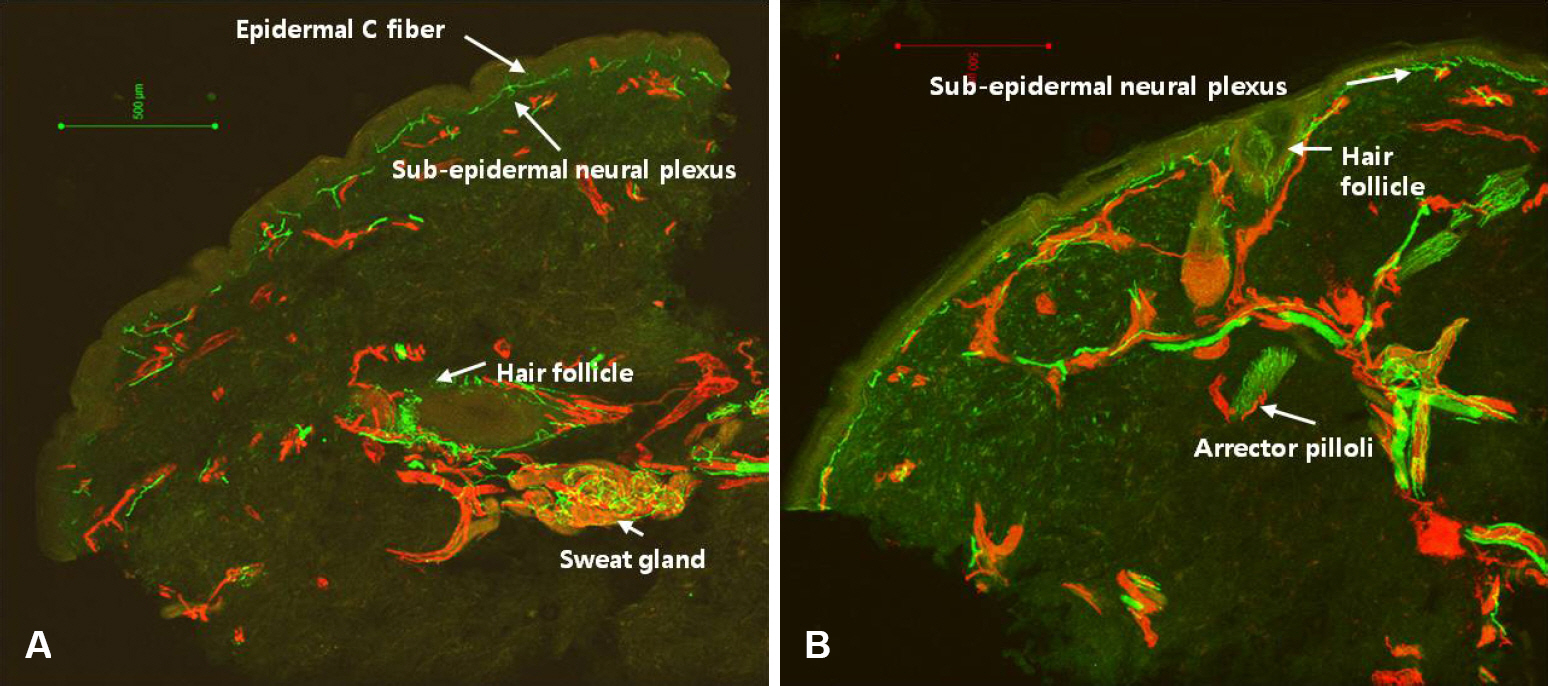
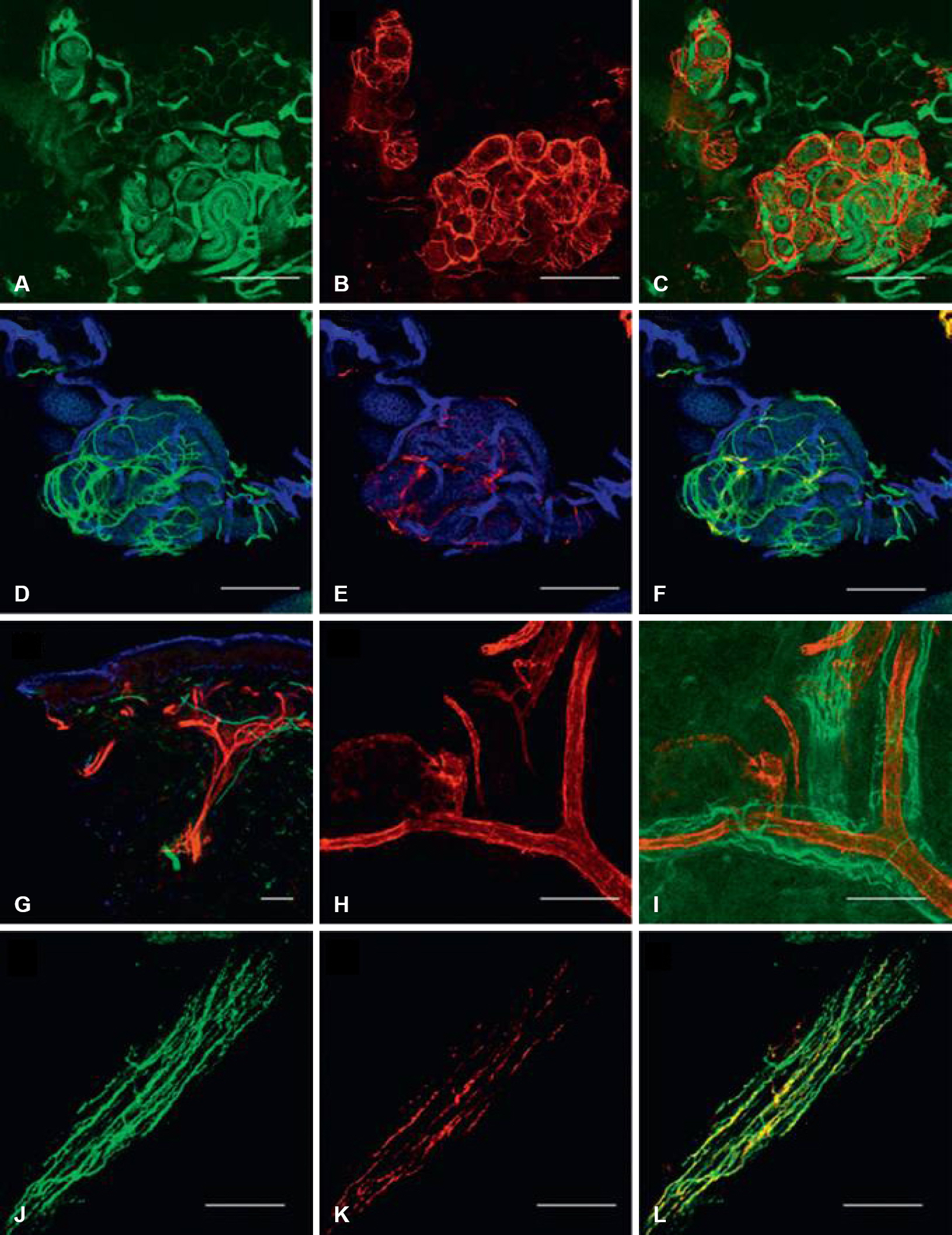
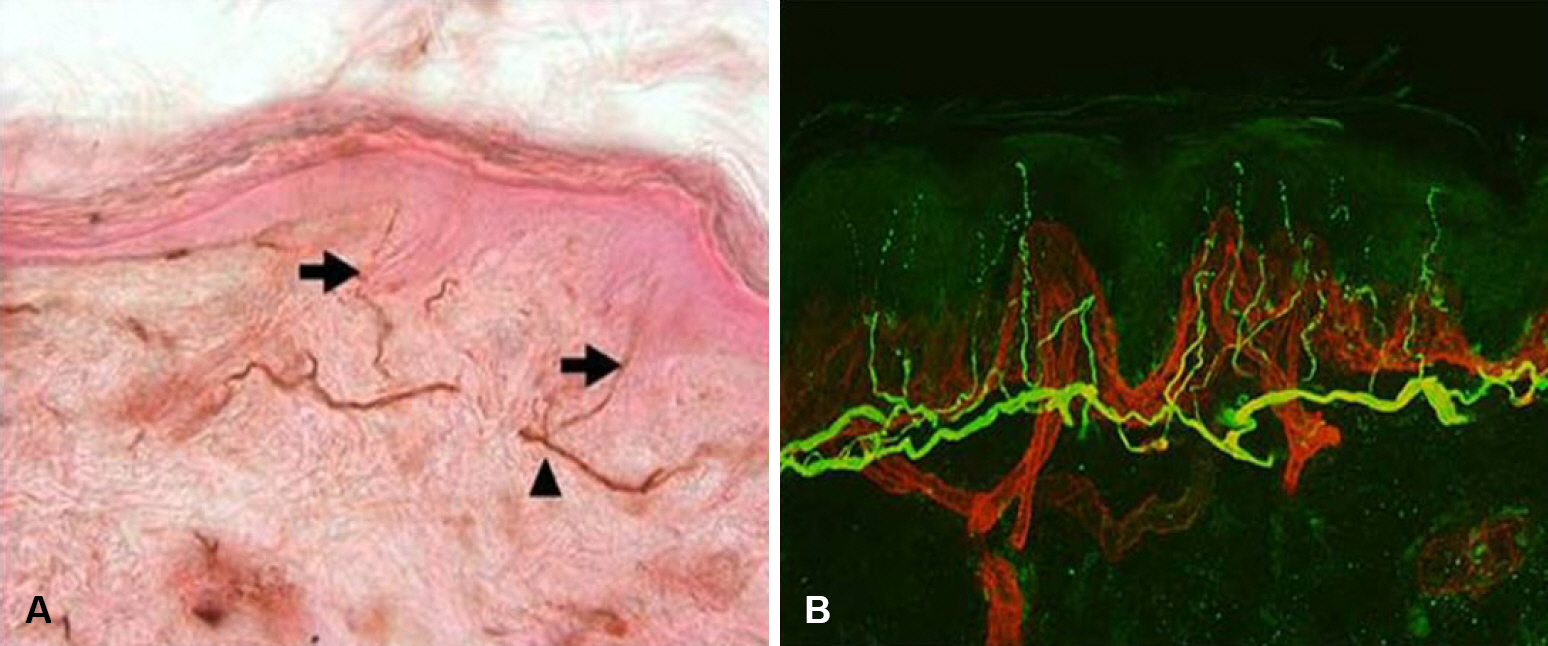
- Skin biopsy with investigation of small nerve fiber in human epidermis and dermis has been proven to be a useful method for demonstration of small fiber neuropathy. Quantification of intraepidermal nerve fiber density using anti-Protein Gene Product 9.5 (PGP 9.5) antibody is standardized method to diagnose the small fiber neuropathy. Skin biopsy method also makes it possible to differentiate the type of nerve fibers by using different antibodies. Quantification of dermal structures with different type of nerve fibers could be used to invest pathophysiologic mechanism of diseased state.
Figure
Reference
-
1.Wang L., Hilliges M., Jernberg T., Wiegleb-Edstrom D., Johansson O. Protein gene product 9.5-immunoreactive nerve fibres and cells in human skin. Cell tissue Res. 1990. 261:25–33.
Article2.Kennedy WR., Wendelschafer-Crabb G. The innervation of human epidermis. J Neurol Sci. 1993. 115:184–190.
Article3.McCarthy BG., Hsieh ST., Stocks A., Hauer P., Macko C., Cornblath DR, et al. Cutaneous innervation in sensory neuropathies: Evaluation by skin biopsy. Neurology. 1995. 45:1848–1855.
Article4.Lauria G., Cornblath DR., Johansson O., McArthur JC., Mellgren SI., Nolano M, et al. Efns guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005. 12:747–758.
Article5.Lauria G., Hsieh ST., Johansson O., Kennedy WR., Leger JM., Mellgren SI, et al. European federation of neurological societies/peripheral nerve society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the european federation of neurological societies and the peripheral nerve society. Eur J Neurol. 2010. 17:903–912. e944-909.6.Wang N., Gibbons CH. Skin biopsies in the assessment of the autonomic nervous system. Handb Clin Neurol. 2013. 117:371–378.
Article7.Braverman IM. The cutaneous microcirculation. J Investig Dermatol Symp Proc. 2000. 5:3–9.
Article8.Mellgren SI., Nolano M., Sommer C. The cutaneous nerve biopsy: Technical aspects, indications, and contribution. Handb Clinical Neurol. 2013. 115:171–188.9.Wang N., Gibbons CH., Freeman R. Novel immunohistochemical techniques using discrete signal amplification systems for human cutaneous peripheral nerve fiber imaging. J Histochem Cytochem. 2011. 59:382–390.
Article10.Pittenger GL., Ray M., Burcus NI., McNulty P., Basta B., Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes care. 2004. 27:1974–1979.
Article11.Chai J., Herrmann DN., Stanton M., Barbano RL., Logigian EL. Painful small-fiber neuropathy in sjogren syndrome. Neurology. 2005. 65:925–927.
Article12.Kennedy WR., Wendelschafer-Crabb G. Utility of skin biopsy in diabetic neuropathy. Semin Neurol. 1996. 16:163–171.
Article13.Di Leo R., Nolano M., Boman H., Pierangeli G., Provitera V., Knappskog PM, et al. Central and peripheral autonomic failure in cold-induced sweating syndrome type 1. Neurology. 2010. 75:1567–1569.
Article14.Donadio V., Nolano M., Elam M., Montagna P., Provitera V., Bugiardini E, et al. Anhidrosis in multiple system atrophy: A preganglionic sudomotor dysfunction? Mov Disord. 2008. 23:885–888.
Article15.Lauria G., Bakkers M., Schmitz C., Lombardi R., Penza P., Devigili G, et al. Intraepidermal nerve fiber density at the distal leg: A worldwide normative reference study. J Peripher Nerv Syst. 2010. 15:202–207.
Article16.Devigili G., Tugnoli V., Penza P., Camozzi F., Lombardi R., Melli G, et al. The diagnostic criteria for small fibre neuropathy: From symptoms to neuropathology. Brain. 2008. 131:1912–1925.
Article17.Nebuchennykh M., Loseth S., Jorde R., Mellgren SI. Idiopathic polyneuropathy and impaired glucose metabolism in a norwegian patient series. Eur J Neurol. 2008. 15:810–816.
Article18.Nolano M., Provitera V., Donadio V., Stancanelli A., Saltalamacchia A., Caporaso G, et al. Ross syndrome: A lesson from a monozygotic twin pair. Neurology. 2013. 80:417–418.
Article19.Nolano M., Provitera V., Perretti A., Stancanelli A., Saltalamacchia AM., Donadio V, et al. Ross syndrome: A rare or a misknown disorder of thermoregulation? A skin innervation study on 12 subjects. Brain. 2006. 129:2119–2131.
Article20.Smith AG., Ramachandran P., Tripp S., Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes- associated neuropathy. Neurology. 2001. 57:1701–1704.21.Sumner CJ., Sheth S., Griffin JW., Cornblath DR., Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003. 60:108–111.
Article22.Chao CC., Hsieh ST., Shun CT., Hsieh SC. Skin denervation and cutaneous vasculitis in eosinophilia-associated neuropathy. Arch Neurol. 2007. 64:959–965.
Article23.Tseng MT., Hsieh SC., Shun CT., Lee KL., Pan CL., Lin WM, et al. Skin denervation and cutaneous vasculitis in systemic lupus erythematosus. Brain. 2006. 129:977–985.
Article24.Nolano M., Manganelli F., Provitera V., Pisciotta C., Stancanelli A., Caporaso G, et al. Small nerve fiber involvement in cmt1a. Neurology. 2015. 84:407–414.
Article25.Sghirlanzoni A., Pareyson D., Lauria G. Sensory neuron diseases. Lancet Neurol. 2005. 4:349–361.
Article26.Kennedy WR., Wendelschafer-Crabb G., Brelje TC. Innervation and vasculature of human sweat glands: An immunohistochemistry-laser scanning confocal fluorescence microscopy study. J Neurosci. 1994. 14:6825–6833.
Article27.Dabby R., Djaldetti R., Shahmurov M., Treves TA., Gabai B., Melamed E, et al. Skin biopsy for assessment of autonomic denervation in parkinson's disease. J Neural transm (Vienna). 2006. 113:1169–1176.
Article28.Dabby R., Vaknine H., Gilad R., Djaldetti R., Sadeh M. Evaluation of cutaneous autonomic innervation in idiopathic sensory small-fiber neuropathy. J Peripher Nerv Syst. 2007. 12:98–101.
Article29.Gibbons CH., Illigens BM., Wang N., Freeman R. Quantification of sudomotor innervation: A comparison of three methods. Muscle Nerve. 2010. 42:112–119.
Article30.Gibbons CH., Illigens BM., Wang N., Freeman R. Quantification of sweat gland innervation: A clinical-pathologic correlation. Neurology. 2009. 72:1479–1486.
Article31.Tesfaye S., Boulton AJ., Dyck PJ., Freeman R., Horowitz M., Kempler P, et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes care. 2010. 33:2285–2293.
Article32.Nolano M., Provitera V., Caporaso G., Stancanelli A., Vitale DF., Santoro L. Quantification of pilomotor nerves: A new tool to evaluate autonomic involvement in diabetes. Neurology. 2010. 75:1089–1097.
Article33.Sohn E., Gibbons CH., Wang N., Freeman R. Quantitative analysis of human cutaneous vasomotor innervation. Auton Neurosci Basic Clin. 2015. 192:114.
Article34.Beach TG., White CL., Hamilton RL., Duda JE., Iwatsubo T., Dickson DW, et al. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta neuropathol. 2008. 116:277–288.35.Shannon KM., Keshavarzian A., Mutlu E., Dodiya HB., Daian D., Jaglin JA, et al. Alpha-synuclein in colonic submucosa in early untreated parkinson's disease. Mov Disord. 2012. 27:709–715.
Article36.Pouclet H., Lebouvier T., Coron E., des Varannes SB., Rouaud T., Roy M, et al. A comparison between rectal and colonic biopsies to detect lewy pathology in parkinson's disease. Neurobiol Dis. 2012. 45:305–309.
Article37.Orimo S., Uchihara T., Nakamura A., Mori F., Kakita A., Wakabayashi K, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in parkinson's disease. Brain. 2008. 131:642–650.38.Nolano M., Provitera V., Estraneo A., Selim MM., Caporaso G., Stancanelli A, et al. Sensory deficit in parkinson's disease: Evidence of a cutaneous denervation. Brain. 2008. 131:1903–1911.
Article39.Wang N., Gibbons CH., Lafo J., Freeman R. Alpha-synuclein in cutaneous autonomic nerves. Neurology. 2013. 81:1604–1610.40.Schneider SA., Boettner M., Alexoudi A., Zorenkov D., Deuschl G., Wedel T. Can we use peripheral tissue biopsies to diagnose parkinson's disease? A review of the literature. Eur J Neurol. 2015.
Article41.Shin RK., Galetta SL., Ting TY., Armstrong K., Bird SJ. Ross syndrome plus: Beyond horner, holmes-adie, and harlequin. Neurology. 2000. 55:1841–1846.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Skin biopsy: an emerging method for small nerve fiber evaluation
- Quantitative sudomotor axon reflex test (QSART) as a diagnostic tool of small fiber neuropathy
- Assessment of Intraepidermal Nerve Fiber using Skin Biopsy in Diabetic Polyneuropathy
- Reproducibility of Retinal Nerve Fiber Layer Thickness Evaluation by Nerve Fiber Analyzer
- Biometry of Retinal Nerve Fiber Layer Thickness by NFA