Korean J Radiol.
2019 Jul;20(7):1114-1123. 10.3348/kjr.2018.0932.
Microvascular Flow Imaging of Residual or Recurrent Hepatocellular Carcinoma after Transarterial Chemoembolization: Comparison with Color/Power Doppler Imaging
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul, Korea. jmsh@snu.ac.kr
- 2Department of Radiology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Radiology, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 4Samsung Medison Co., Ltd., Seoul, Korea.
- 5Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul, Korea.
- KMID: 2467022
- DOI: http://doi.org/10.3348/kjr.2018.0932
Abstract
OBJECTIVE
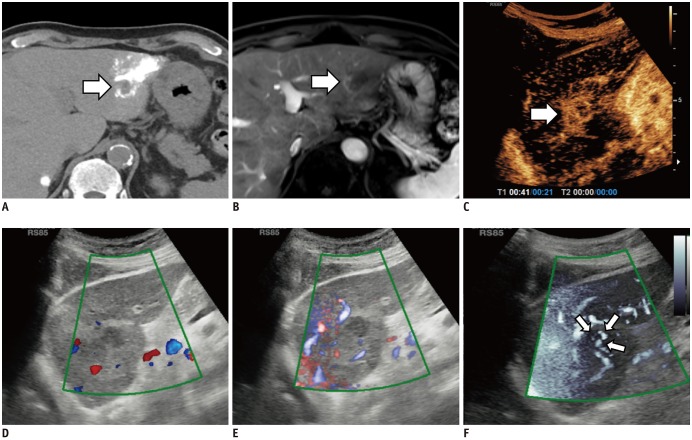
To determine the feasibility of microvascular flow imaging (MVFI) in comparison with color/power Doppler imaging (CDI/PDI) for detection of intratumoral vascularity in suspected post-transarterial chemoembolization (TACE) residual or recurrent hepatocellular carcinomas (HCCs) by using contrast-enhanced ultrasonography (CEUS) or hepatic angiography (HA) findings as the reference standard.
MATERIALS AND METHODS
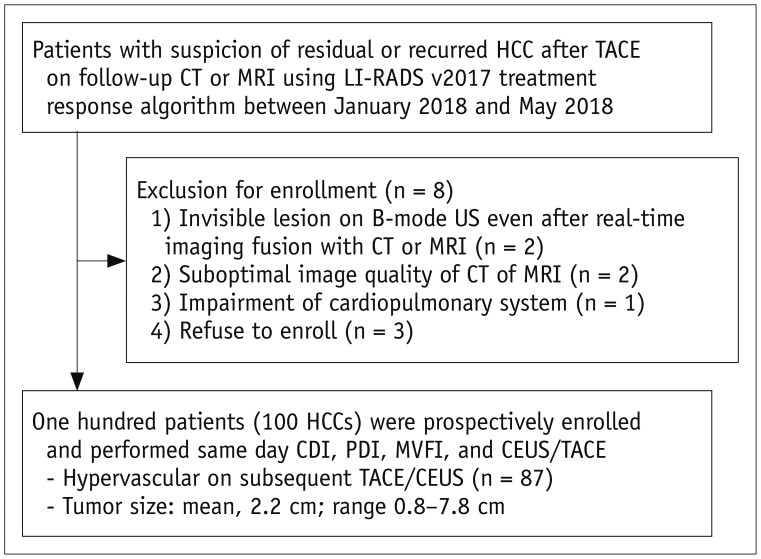
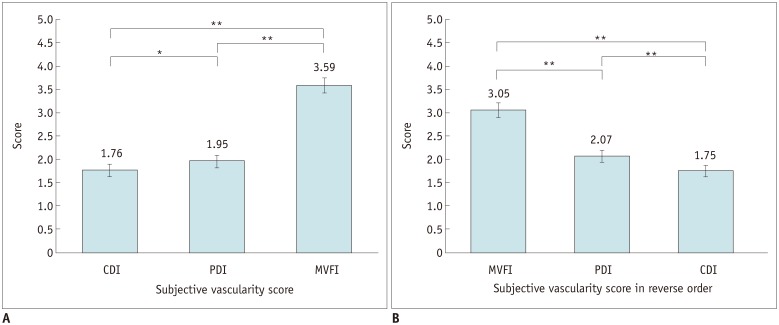
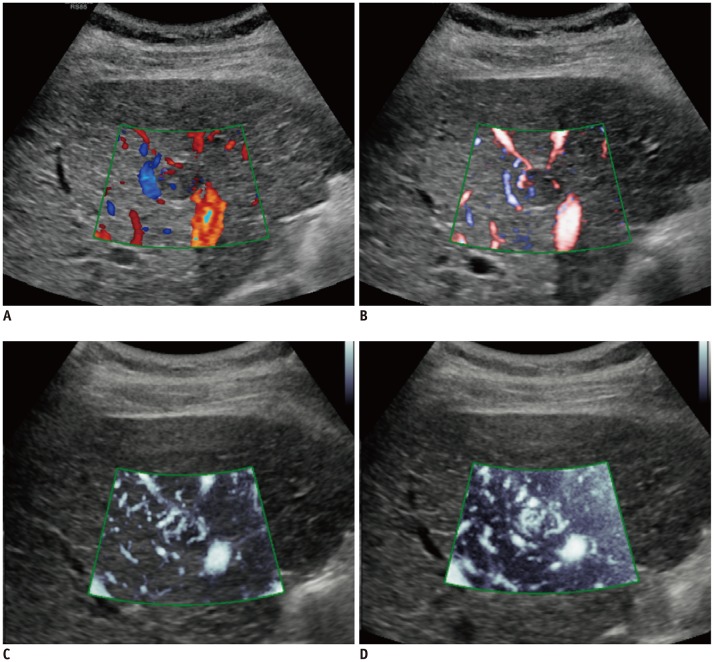
One hundred HCCs (mean size, 2.2 cm) in 100 patients treated with TACE were included in this prospective study. CDI, PDI, and MVFI were performed in tandem for evaluating intratumoral vascularity of the lesions by using an RS85 ultrasound scanner (Samsung Medison Co., Ltd.). Intratumoral vascularity in each technique was assessed by two radiologists in consensus by using a 5-point scale. Then, one of the two radiologists and another radiologist performed additional image review in the reverse order (MVFI-PDI-CDI) for evaluation of intra- and interobserver agreements. Results were then compared with those of either HA or CEUS as the reference. The McNemar test, logistic regression analysis, and intraclass correlation coefficient (ICC) were used.
RESULTS
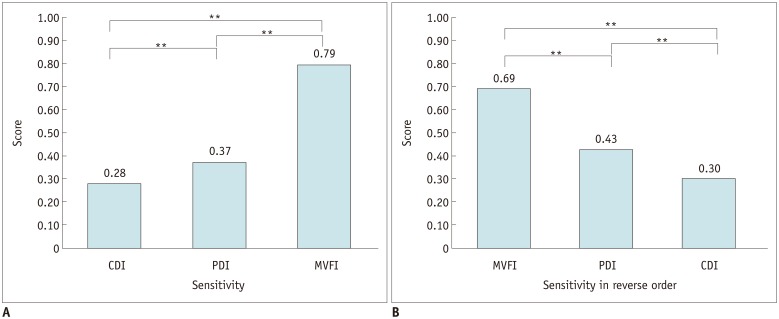
CEUS or HA revealed intratumoral vascularity in 87% (87/100) of the tumors. Sensitivity (79.3%, 69/87) and accuracy (80.0%, 80/100) of MVFI were significantly higher than those of CDI (sensitivity, 27.6% [24/87]; accuracy, 37.0% [37/100]) or PDI (sensitivity, 36.8% [32/87]; accuracy, 44.0% [44/100]) (all p < 0.05). CDI, PDI, and MVFI presented excellent intraobserver (ICCs > 0.9) and good interobserver agreements (ICCs > 0.6).
CONCLUSION
MVFI demonstrated significantly higher sensitivity and accuracy than did CDI and PDI for the detection of intratumoral vascularity in suspected residual or recurrent HCCs after TACE.
Keyword
MeSH Terms
Figure
Reference
-
1. Mittelstaedt CA. Ultrasound as a useful imaging modality for tumor detection and staging. Cancer Res. 1980; 40(8 Pt 2):3072–3078. PMID: 7397702.2. Wernecke K, Vassallo P, Bick U, Diederich S, Peters PE. The distinction between benign and malignant liver tumors on sonography: value of a hypoechoic halo. AJR Am J Roentgenol. 1992; 159:1005–1009. PMID: 1329454.
Article3. Strobel D, Seitz K, Blank W, Schuler A, Dietrich C, von Herbay A, et al. Contrast-enhanced ultrasound for the characterization of focal liver lesions--diagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall Med. 2008; 29:499–505. PMID: 19241506.4. D'Onofrio M, Crosara S, De Robertis R, Canestrini S, Mucelli RP. Contrast-enhanced ultrasound of focal liver lesions. AJR Am J Roentgenol. 2015; 205:W56–W66. PMID: 26102419.5. Choi BI, Kim HC, Han JK, Park JH, Kim YI, Kim ST, et al. Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology. 1992; 182:709–713. PMID: 1311116.
Article6. Cioni D, Lencioni R, Bartolozzi C. Therapeutic effect of transcatheter arterial chemoembolization on hepatocellular carcinoma: evaluation with contrast-enhanced harmonic power Doppler ultrasound. Eur Radiol. 2000; 10:1570–1575. PMID: 11044926.
Article7. Tanaka S, Kitamra T, Fujita M, Kasugai H, Inoue A, Ishiguro S. Small hepatocellular carcinoma: differentiation from adenomatous hyperplastic nodule with color Doppler flow imaging. Radiology. 1992; 182:161–165. PMID: 1309217.
Article8. Numata K, Tanaka K, Kiba T, Morimoto M, Arata S, Kondo M, et al. Use of hepatic tumor index on color Doppler sonography for differentiating large hepatic tumors. AJR Am J Roentgenol. 1997; 168:991–995. PMID: 9124156.
Article9. Tanaka K, Inoue S, Numata K, Takamura Y, Takebayashi S, Ohaki Y, et al. Color Doppler sonography of hepatocellular carcinoma before and after treatment by transcatheter arterial embolization. AJR Am J Roentgenol. 1992; 158:541–546. PMID: 1310826.
Article11. Yoo YM, Managuli R, Kim Y. Adaptive clutter filtering for ultrasound color flow imaging. Ultrasound Med Biol. 2003; 29:1311–1320. PMID: 14553809.
Article12. Yoo YM, Kim Y. New adaptive clutter rejection for ultrasound color Doppler imaging: in vivo study. Ultrasound Med Biol. 2010; 36:480–487. PMID: 20133045.
Article13. Moschouris H, Kalokairinou-Motogna M, Vrakas S, Papadatou A, Karagiannis E, Kiltenis M, et al. Imaging of intrahepatic progression of hepatocellular carcinoma post transarterial chemoembolization. A long-term, prospective evaluation of contrast-enhanced ultrasonography (CEUS). Med Ultrason. 2017; 19:134–142. PMID: 28440346.
Article14. Wobser H, Wiest R, Salzberger B, Wohlgemuth WA, Stroszczynski C, Jung EM. Evaluation of treatment response after chemoembolisation (TACE) in hepatocellular carcinoma using real time image fusion of contrast-enhanced ultrasound (CEUS) and computed tomography (CT)--preliminary results. Clin Hemorheol Microcirc. 2014; 57:191–201. PMID: 24577382.15. Salvaggio G, Campisi A, Lo Greco V, Cannella I, Meloni MF, Caruso G. Evaluation of posttreatment response of hepatocellular carcinoma: comparison of ultrasonography with second-generation ultrasound contrast agent and multidetector CT. Abdom Imaging. 2010; 35:447–453. PMID: 19562414.
Article16. Kang HJ, Kim JH, Lee SM, Yang HK, Ahn SJ, Han JK. Additional value of contrast-enhanced ultrasonography for fusion-guided, percutaneous biopsies of focal liver lesions: prospective feasibility study. Abdom Radiol (NY). 2018; 43:3279–3287. PMID: 29671007.
Article17. Park AY, Seo BK. Up-to-date Doppler techniques for breast tumor vascularity: superb microvascular imaging and contrastenhanced ultrasound. Ultrasonography. 2018; 37:98–106. PMID: 29025210.
Article18. Ma Y, Li G, Li J, Ren WD. The diagnostic value of superb microvascular imaging (SMI) in detecting blood flow signals of breast lesions: a preliminary study comparing SMI to color Doppler flow imaging. Medicine (Baltimore). 2015; 94:e1502. PMID: 26356718.19. Machado P, Segal S, Lyshchik A, Forsberg F. A novel microvascular flow technique: initial results in thyroids. Ultrasound Q. 2016; 32:67–74. PMID: 25900162.20. Lee YS, Kim MJ, Han SW, Lee HS, Im YJ, Shin HJ, et al. Superb microvascular imaging for the detection of parenchymal perfusion in normal and undescended testes in young children. Eur J Radiol. 2016; 85:649–656. PMID: 26860680.
Article21. Dubinsky TJ, Revels J, Wang S, Toia G, Sonneborn R, Hippe DS, et al. Comparison of superb microvascular imaging with color flow and power Doppler imaging of small hepatocellular carcinomas. J Ultrasound Med. 2018; 37:2915–2924. PMID: 29683199.
Article22. He MN, Lv K, Jiang YX, Jiang TA. Application of superb microvascular imaging in focal liver lesions. World J Gastroenterol. 2017; 23:7765–7775. PMID: 29209117.
Article23. Elsayes KM, Hooker JC, Agrons MM, Kielar AZ, Tang A, Fowler KJ, et al. 2017 version of LI-RADS for CT and MR imaging: an update. Radiographics. 2017; 37:1994–2017. PMID: 29131761.
Article24. Kremkau FW, Taylor KJ. Artifacts in ultrasound imaging. J Ultrasound Med. 1986; 5:227–237. PMID: 3514956.
Article25. Lin DC, Nazarian LN, O'Kane PL, McShane JM, Parker L, Merritt CR. Advantages of real-time spatial compound sonography of the musculoskeletal system versus conventional sonography. AJR Am J Roentgenol. 2002; 179:1629–1631. PMID: 12438067.26. Kang HJ, Kim YI, Kim HC, Jae HJ, Hur S, Chung JW. Does establishing a safety margin reduce local recurrence in subsegmental transarterial chemoembolization for small nodular hepatocellular carcinomas? Korean J Radiol. 2015; 16:1068–1078. PMID: 26357501.
Article27. Rubin JM, Bude RO, Carson PL, Bree RL, Adler RS. Power Doppler US: a potentially useful alternative to mean frequency-based color Doppler US. Radiology. 1994; 190:853–856. PMID: 8115639.
Article28. Kubota K, Hisa N, Fujiwara Y, Fukumoto M, Yoshida D, Yoshida S. Evaluation of the intratumoral vasculature of hepatocellular carcinoma by power Doppler sonography: advantages and disadvantages versus conventional color Doppler sonography. Abdom Imaging. 2000; 25:172–178. PMID: 10675460.
Article29. Oosterveld BJ, Thijssen JM, Hartman PC, Romijn RL, Rosenbusch GJ. Ultrasound attenuation and texture analysis of diffuse liver disease: methods and preliminary results. Phys Med Biol. 1991; 36:1039–1064. PMID: 1924541.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Power Doppler and Color Doppler Ultrasonography in the Detection of Intratesticular Blood Flow of Normal Infants
- Three-Dimensional Power Doppler Imaging
- Ultrasonographic Demonstration of the Tissue Microvasculature in Children: Microvascular Ultrasonography Versus Conventional Color Doppler Ultrasonography
- Complications Related to Transarterial Treatment of Hepatocellular Carcinoma: A Comprehensive Review
- Color doppler echocardiographic evaluation of residual ductal flow after surgical ligation