Korean J Physiol Pharmacol.
2020 Jan;24(1):53-68. 10.4196/kjpp.2020.24.1.53.
Interaction of genetic background and exercise training intensity on endothelial function in mouse aorta
- Affiliations
-
- 1Department of Sports Science, Seoul National University of Science and Technology, Seoul 01811, Korea. skkim7@seoultech.ac.kr
- 2Department of Health and Kinesiology, Texas A&M University, College Station, TX, USA.
- KMID: 2466570
- DOI: http://doi.org/10.4196/kjpp.2020.24.1.53
Abstract
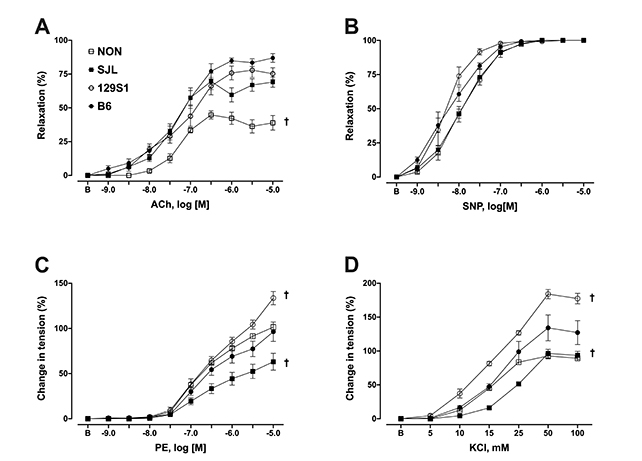
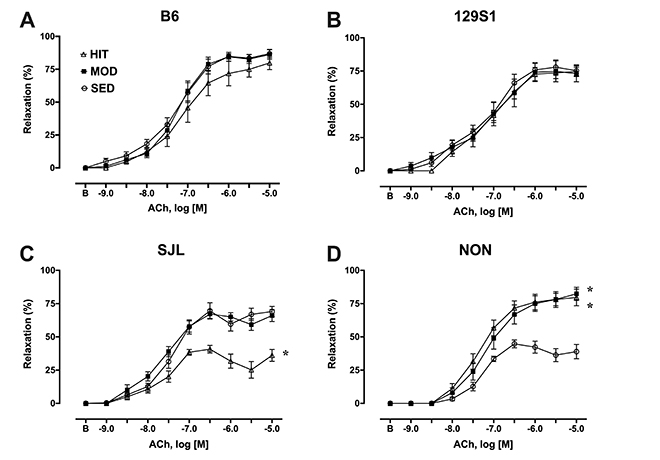
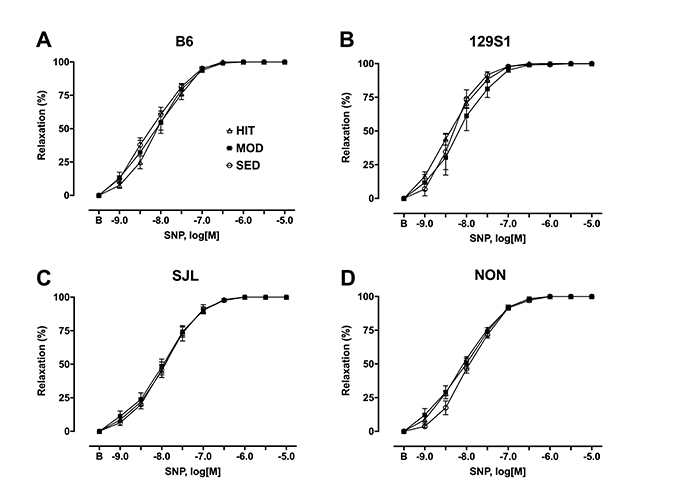
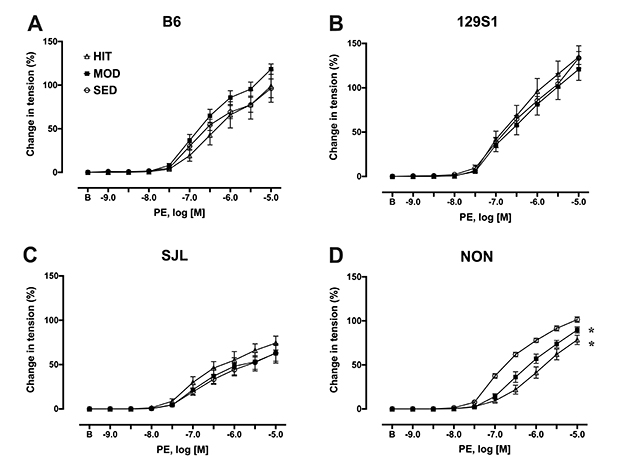
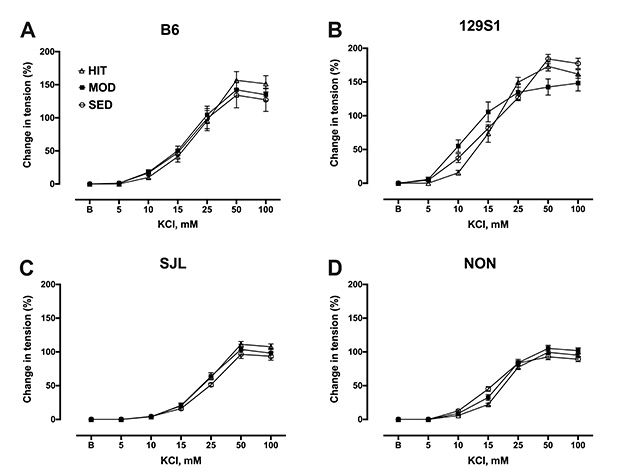
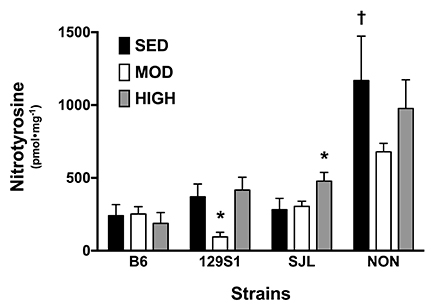
- The purpose of this study was to characterize the genetic contribution to endothelial adaptation to exercise training. Vasoreactivity was assessed in aortas from four inbred mouse strains (129S1, B6, NON, and SJL) after 4 weeks of moderate intensity continuous exercise training (MOD), high intensity interval training (HIT) or in sedentary controls (SED). Intrinsic variations in endothelium-dependent vasorelaxation (EDR) to acetylcholine (ACh) as well as vasocontractile responses were observed across SED groups. For responses to exercise training, there was a significant interaction between mouse strain and training intensity on EDR. Exercise training had no effect on EDR in aortas from 129S1 and B6 mice. In NON, EDR was improved in aortas from MOD and HIT compared with respective SED, accompanied by diminished responses to PE in those groups. Interestingly, EDR was impaired in aorta from SJL HIT compared with SED. The transcriptional activation of endothelial genes was also influenced by the interaction between mouse strain and training intensity. The number of genes altered by HIT was greater than MOD, and there was little overlap between genes altered by HIT and MOD. HIT was associated with gene pathways for inflammatory responses. NON MOD genes showed enrichment for vessel growth pathways. These findings indicate that exercise training has non-uniform effects on endothelial function and transcriptional activation of endothelial genes depending on the interaction between genetic background and training intensity.
MeSH Terms
Figure
Reference
-
1. Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2008; 93:141–147.
Article2. Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol (1985). 1993; 75:1354–1363.
Article3. Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, Wisløff U, Ellingsen Ø. Moderate vs. high exercise intensity: differential effects on aerobic fitness,cardiomyocyte contractility, and endothelial function. Cardiovasc Res. 2005; 67:161–172.4. Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation. 1999; 100:1194–1202.5. Jasperse JL, Laughlin MH. Endothelial function and exercise training: evidence from studies using animal models. Med Sci Sports Exerc. 2006; 38:445–454.6. Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994; 74:349–353.
Article7. Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol. 2000; 279:H2068–H2076.
Article8. Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation. 1994; 89:2308–2314.
Article9. Green DJ, Eijsvogels T, Bouts YM, Maiorana AJ, Naylor LH, Scholten RR, Spaanderman ME, Pugh CJ, Sprung VS, Schreuder T, Jones H, Cable T, Hopman MT, Thijssen DH. Exercise training and artery function in humans: nonresponse and its relationship to cardiovascular risk factors. J Appl Physiol (1985). 2014; 117:345–352.
Article10. Ryan MJ, Didion SP, Davis DR, Faraci FM, Sigmund CD. Endothelial dysfunction and blood pressure variability in selected inbred mouse strains. Arterioscler Thromb Vasc Biol. 2002; 22:42–48.
Article11. Chen C, Korshunov VA, Massett MP, Yan C, Berk BC. Impaired vasorelaxation in inbred mice is associated with alterations in both nitric oxide and super oxide pathways. J Vasc Res. 2007; 44:504–512.
Article12. Kim SK, Avila JJ, Massett MP. Strain survey and genetic analysis of vasoreactivity in mouse aorta. Physiol Genomics. 2016; 48:861–873.
Article13. Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF Jr, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004; 109:613–619.14. Zhao J, Cheema FA, Reddy U, Bremner JD, Su S, Goldberg J, Snieder H, Vaccarino V. Heritability of flow-mediated dilation: a twin study. J Thromb Haemost. 2007; 5:2386–2392.
Article15. Suzuki K, Juo SH, Rundek T, Boden-Albala B, Disla N, Liu R, Park N, Di Tullio MR, Sacco RL, Homma S. Genetic contribution to brachial artery flow-mediated dilation: the Northern Manhattan Family Study. Atherosclerosis. 2008; 197:212–216.
Article16. Kim SK, Massett MP. Genetic regulation of endothelial vasomotor function. Front Physiol. 2016; 7:571.
Article17. Hopkins N, Stratton G, Maia J, Tinken TM, Graves LE, Cable TN, Green DJ. Heritability of arterial function, fitness, and physical activity in youth: a study of monozygotic and dizygotic twins. J Pediatr. 2010; 157:943–948.
Article18. Hopkins ND, Stratton G, Cable NT, Tinken TM, Graves LE, Green DJ. Impact of exercise training on endothelial function and body composition in young people: a study of mono- and di-zygotic twins. Eur J Appl Physiol. 2012; 112:421–427.
Article19. Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006; 97:141–147.
Article20. Helgerud J, Høydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007; 39:665–671.
Article21. Høydal MA, Wisløff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007; 14:753–760.
Article22. Chung E, Diffee GM. Moderate intensity, but not high intensity, treadmill exercise training alters power output properties in myocardium from aged rats. J Gerontol A Biol Sci Med Sci. 2012; 67:1178–1187.
Article23. Hafstad AD, Lund J, Hadler-Olsen E, Höper AC, Larsen TS, Aasum E. High- and moderate-intensity training normalizes ventricular function and mechanoenergetics in mice with diet-induced obesity. Diabetes. 2013; 62:2287–2294.
Article24. Rognmo Ø, Moholdt T, Bakken H, Hole T, Mølstad P, Myhr NE, Grimsmo J, Wisløff U. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. 2012; 126:1436–1440.
Article25. Bergholm R, Mäkimattila S, Valkonen M, Liu ML, Lahdenperä S, Taskinen MR, Sovijärvi A, Malmberg P, Yki-Järvinen H. Intense physical training decreases circulating antioxidants and endothelium-dependent vasodilatation in vivo. Atherosclerosis. 1999; 145:341–349.26. Iemitsu M, Miyauchi T, Maeda S, Yuki K, Kobayashi T, Kumagai Y, Shimojo N, Yamaguchi I, Matsuda M. Intense exercise causes decrease in expression of both endothelial NO synthase and tissue NOx level in hearts. Am J Physiol Regul Integr Comp Physiol. 2000; 279:R951–R959.
Article27. Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003; 108:530–535.28. Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC, Brugada J, Nattel S, Mont L. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011; 123:13–22.
Article29. Haram PM, Kemi OJ, Lee SJ, Bendheim MØ, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisløff U. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res. 2009; 81:723–732.
Article30. Murias JM, Dey A, Campos OA, Estaki M, Hall KE, Melling CW, Noble EG. High-intensity endurance training results in faster vessel-specific rate of vasorelaxation in type 1 diabetic rats. PLoS One. 2013; 8:e59678.
Article31. Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008; 118:346–354.32. Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ, MacDonald MJ. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2008; 295:R236–R242.
Article33. Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004; 14:1806–1811.
Article34. Avila JJ, Kim SK, Massett MP. Differences in exercise capacity and responses to training in 24 inbred mouse strains. Front Physiol. 2017; 8:974.
Article35. Massett MP, Avila JJ, Kim SK. Exercise capacity and response to training quantitative trait loci in a NZW X 129S1 intercross and combined cross analysis of inbred mouse strains. PLoS One. 2015; 10:e0145741.
Article36. Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc. 2012; 7:1235–1246.
Article37. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000; 87:840–844.
Article38. Seals DR, Edward F. Adolph distinguished lecture: the remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol (1985). 2014; 117:425–439.39. Prochazka M, Serreze DV, Worthen SM, Leiter EH. Genetic control of diabetogenesis in NOD/Lt mice. Development and analysis of congenic stocks. Diabetes. 1989; 38:1446–1455.
Article40. Svenson KL, Von Smith R, Magnani PA, Suetin HR, Paigen B, Naggert JK, Li R, Churchill GA, Peters LL. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J Appl Physiol (1985). 2007; 102:2369–2378.
Article41. Hoshino J, Sakamaki T, Nakamura T, Kobayashi M, Kato M, Sakamoto H, Kurashina T, Yagi A, Sato K, Ono Z. Exaggerated vascular response due to endothelial dysfunction and role of the renin-angiotensin system at early stage of renal hypertension in rats. Circ Res. 1994; 74:130–138.
Article42. Durand MJ, Gutterman DD. Exercise and vascular function: how much is too much? Can J Physiol Pharmacol. 2014; 92:551–557.
Article43. Delp MD, Laughlin MH. Time course of enhanced endothelium-mediated dilation in aorta of trained rats. Med Sci Sports Exerc. 1997; 29:1454–1461.
Article44. Green DJ, Cable NT, Fox C, Rankin JM, Taylor RR. Modification of forearm resistance vessels by exercise training in young men. J Appl Physiol (1985). 1994; 77:1829–1833.
Article45. Padilla J, Newcomer SC, Simmons GH, Kreutzer KV, Laughlin MH. Long-term exercise training does not alter brachial and femoral artery vasomotor function and endothelial phenotype in healthy pigs. Am J Physiol Heart Circ Physiol. 2010; 299:H379–H385.
Article46. Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol (1985). 2009; 106:1925–1934.
Article47. Johnson LR, Laughlin MH. Chronic exercise training does not alter pulmonary vasorelaxation in normal pigs. J Appl Physiol (1985). 2000; 88:2008–2014.48. Batacan RB Jr, Duncan MJ, Dalbo VJ, Connolly KJ, Fenning AS. Light-intensity and high-intensity interval training improve cardiometabolic health in rats. Appl Physiol Nutr Metab. 2016; 41:945–952.
Article49. Molmen-Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA, Wisloff U, Ingul CB, Stoylen A. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012; 19:151–160.
Article50. Wang JS. Intense exercise increases shear-induced platelet aggregation in men through enhancement of von Willbrand factor binding, glycoprotein IIb/IIIa activation, and P-selectin expression on platelets. Eur J Appl Physiol. 2004; 91:741–747.
Article51. Pereira BC, Filho LA, Alves GF, Pauli JR, Ropelle ER, Souza CT, Cintra DE, Saad MJ, Silva AS. A new overtraining protocol for mice based on downhill running sessions. Clin Exp Pharmacol Physiol. 2012; 39:793–798.
Article52. Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. Transcriptome-wide RNA sequencing analysis of rat skeletal muscle feed arteries. II. Impact of exercise training in obesity. J Appl Physiol (1985). 2014; 116:1033–1104.
Article53. Padilla J, Simmons GH, Davis JW, Whyte JJ, Zderic TW, Hamilton MT, Bowles DK, Laughlin MH. Impact of exercise training on endothelial transcriptional profiles in healthy swine: a genome-wide microarray analysis. Am J Physiol Heart Circ Physiol. 2011; 301:H555–H564.
Article54. Palmefors H, DuttaRoy S, Rundqvist B, Börjesson M. The effect of physical activity or exercise on key biomarkers in atherosclerosis--a systematic review. Atherosclerosis. 2014; 235:150–161.55. Prior BM, Lloyd PG, Yang HT, Terjung RL. Exercise-induced vascular remodeling. Exerc Sport Sci Rev. 2003; 31:26–33.
Article56. Ratkevicius A, Carroll AM, Kilikevicius A, Venckunas T, McDermott KT, Gray SR, Wackerhage H, Lionikas A. H55N polymorphism as a likely cause of variation in citrate synthase activity of mouse skeletal muscle. Physiol Genomics. 2010; 42A:96–102.
Article57. Laughlin MH, Welshons WV, Sturek M, Rush JW, Turk JR, Taylor JA, Judy BM, Henderson KK, Ganjam VK. Gender, exercise training, and eNOS expression in porcine skeletal muscle arteries. J Appl Physiol (1985). 2003; 95:250–264.58. Laughlin MH, Schrage WG, McAllister RM, Garverick HA, Jones AW. Interaction of gender and exercise training: vasomotor reactivity of porcine skeletal muscle arteries. J Appl Physiol (1985). 2001; 90:216–227.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Aerobic/Resistance Exercise on Body Fat Mass, Muscle Strength and Endothelial Function in Korean Type 2 Diabetes mellitus Patients
- The Effect of Combined Training at Different Times of Day on Body Composition, Plasma Lipids, Stress Hormones and Nutrient Intakes
- Effects of Exercise Training on Vascular Endothelial Function Related Factors of Obese Elderly Women with Sarcopenia
- Effects of Aerobic Exercise vs. Resistance Training on Endothelial Function in Women with Type 2 Diabetes Mellitus
- Genetic approaches toward understanding the individual variation in cardiac structure, function and responses to exercise training