Infect Chemother.
2019 Dec;51(4):365-375. 10.3947/ic.2019.51.4.365.
Characteristics of Faecal Microbiota in Korean Patients with Clostridioides difficile-associated Diarrhea
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. seran@yuhs.ac
- 2AIDS Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea.
- 4Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 5Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2466469
- DOI: http://doi.org/10.3947/ic.2019.51.4.365
Abstract
- BACKGROUND
The intestinal microbiota plays an important role in the pathogenesis of Clostridioides difficile-associated diarrhea, and regional and racial characteristics influence the microbiome composition and diversity. We investigated the intestinal microbiome characteristics of patients with C. difficile colitis (CD+) compared to those of patients with colitis not due to C. difficile (CD−), patients with vancomycin-resistant enterococci (VRE) colonization, and healthy controls, in Korea.
MATERIALS AND METHODS
We collected stool samples from 24, 18, 11 and 13 subjects within CD+, CD−, VRE and healthy control groups, respectively. The microbial communities were evaluated by 454-pyrosequencing of bacterial 16s rRNA.
RESULTS
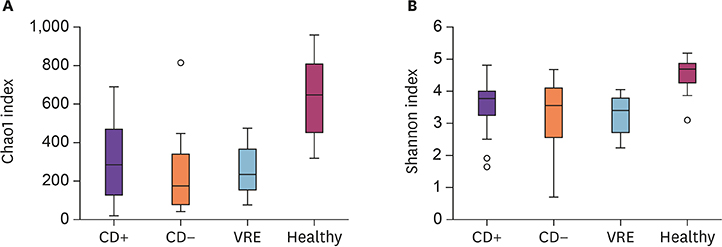
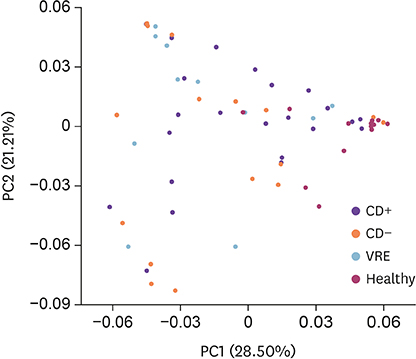
The species richness and microbial diversity were significantly lower in the CD+ group compared to those in healthy controls, but not compared to those in CD− and VRE groups. Phylum-level analysis showed that the proportion of Actinobacteria in the CD+ group was significantly lower than in the healthy control, but was unchanged compared to that in CD− and VRE groups. At the genus level, compared to the healthy group, the CD+ group showed significantly lower proportions of Blautia, Bifidobacterium, Faecalibacterium et al. Compared to the VRE group, the CD+ group showed a significantly higher proportion of Anaerostipes.
CONCLUSIONS
We could identify the intestinal microbiome characteristics of Koreans with C. difficile colitis. It might help to develop microbiome based diagnostic and treatment modalities.
Keyword
MeSH Terms
Figure
Reference
-
1. Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002; 22:283–307.
Article2. Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Med. 2011; 3:14.
Article3. Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft HJ, Doré J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006; 72:1027–1033.
Article4. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012; 486:222–227.
Article5. Dave M, Higgins PD, Middha S, Rioux KP. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012; 160:246–257.
Article6. Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J Gastroenterol. 2016; 22:2219–2241.
Article7. Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, Stanton C. Gut microbiota, obesity and diabetes. Postgrad Med J. 2016.
Article8. Singh V, Yeoh BS, Vijay-Kumar M. Gut microbiome as a novel cardiovascular therapeutic target. Curr Opin Pharmacol. 2016; 27:8–12.
Article9. Seekatz AM, Young VB. Clostridium difficile and the microbiota. J Clin Invest. 2014; 124:4182–4189.
Article10. Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008; 359:1932–1940.11. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002; 97:1769–1775.
Article12. van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile . N Engl J Med. 2013; 368:407–415.
Article13. Almeida R, Gerbaba T, Petrof EO. Recurrent Clostridium difficile infection and the microbiome. J Gastroenterol. 2016; 51:1–10.
Article14. Rupnik M. Toward a true bacteriotherapy for Clostridium difficile infection. N Engl J Med. 2015; 372:1566–1568.
Article15. Moon KR, Sohn KM, Park BM, Kim YS, Chun S, Jung H, Song CH. Successful fecal transplantation by enema for recurrent and refractory Clostridium difficile infection. J Korean Geriatr Soc. 2013; 17:152–156.
Article16. Kook SY, Kim Y, Kang B, Choe YH, Kim YH, Kim S. Characterization of the fecal microbiota differs between age groups in Koreans. Intest Res. 2018; 16:246–254.
Article17. Shin JH, Jung S, Kim SA, Kang MS, Kim MS, Joung H, Hwang GS, Shin DM. Differential Effects of Typical Korean Versus American-Style Diets on Gut Microbial Composition and Metabolic Profile in Healthy Overweight Koreans: A Randomized Crossover Trial. Nutrients. 2019; 11:pii: E2450.
Article18. Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002; 136:834–844.
Article19. Özsoy S, İlki A. Detection of vancomycin-resistant enterococci (VRE) in stool specimens submitted for Clostridium difficile toxin testing. Braz J Microbiol. 2017; 48:489–492.
Article20. Ray AJ, Hoyen CK, Das SM, Eckstein EC, Donskey CJ. Undetected vancomycin-resistant Enterococcus stool colonization in a Veterans Affairs Hospital using a Clostridium difficile-focused surveillance strategy. Infect Control Hosp Epidemiol. 2002; 23:474–477.
Article21. Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile . Clin Infect Dis. 2002; 34:346–353.
Article22. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007; 45:302–307.
Article23. Kato H, Kato N, Watanabe K, Iwai N, Nakamura H, Yamamoto T, Suzuki K, Kim SM, Chong Y, Wasito EB. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J Clin Microbiol. 1998; 36:2178–2182.
Article24. Kwon SS, Gim JL, Kim MS, Kim H, Choi JY, Yong D, Lee K. Clinical and molecular characteristics of community-acquired Clostridium difficile infections in comparison with those of hospital-acquired C. difficile . Anaerobe. 2017; 48:42–46.
Article25. Chun J, Kim KY, Lee JH, Choi Y. The analysis of oral microbial communities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiol. 2010; 10:101.
Article26. Hur M, Kim Y, Song HR, Kim JM, Choi YI, Yi H. Effect of genetically modified poplars on soil microbial communities during the phytoremediation of waste mine tailings. Appl Environ Microbiol. 2011; 77:7611–7619.
Article27. Kim BS, Kim JN, Yoon SH, Chun J, Cerniglia CE. Impact of enrofloxacin on the human intestinal microbiota revealed by comparative molecular analysis. Anaerobe. 2012; 18:310–320.
Article28. Huber T, Faulkner G, Hugenholtz P. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics. 2004; 20:2317–2319.
Article29. Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012; 62:716–721.
Article30. Hamady M, Lozupone C, Knight R. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J. 2010; 4:17–27.
Article31. Milani C, Ticinesi A, Gerritsen J, Nouvenne A, Lugli GA, Mancabelli L, Turroni F, Duranti S, Mangifesta M, Viappiani A, Ferrario C, Maggio M, Lauretani F, De Vos W, van Sinderen D, Meschi T, Ventura M. Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: a metagenomic study. Sci Rep. 2016; 6:25945.
Article32. Zhang L, Dong D, Jiang C, Li Z, Wang X, Peng Y. Insight into alteration of gut microbiota in Clostridium difficile infection and asymptomatic C. difficile colonization. Anaerobe. 2015; 34:1–7.
Article33. Han SH, Yi J, Kim JH, Lee S, Moon HW. Composition of gut microbiota in patients with toxigenic Clostridioides (Clostridium) difficile: Comparison between subgroups according to clinical criteria and toxin gene load. PLoS One. 2019; 14:e0212626.34. Donskey CJ, Chowdhry TK, Hecker MT, Hoyen CK, Hanrahan JA, Hujer AM, Hutton-Thomas RA, Whalen CC, Bonomo RA, Rice LB. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med. 2000; 343:1925–1932.
Article35. Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, Pamer EG. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010; 120:4332–4341.
Article36. Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013; 145:946–953.
Article37. Sohn KM, Cheon S, Kim YS. Can Fecal microbiota transplantation (FMT) eradicate fecal colonization with vancomycin-resistant enterococci (VRE)? Infect Control Hosp Epidemiol. 2016; 37:1519–1521.
Article38. Manges AR, Steiner TS, Wright AJ. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: a review. Infect Dis (Lond). 2016; 48:587–592.
Article39. Bar-Yoseph H, Hussein K, Braun E, Paul M. Natural history and decolonization strategies for ESBL/carbapenem-resistant Enterobacteriaceae carriage: systematic review and meta-analysis. J Antimicrob Chemother. 2016; 71:2729–2739.
Article40. Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MR, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile . Nature. 2015; 517:205–208.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Helicobacter pylori Eradication Therapy-associated Diarrhea
- Why is it so difficult to evaluate faecal microbiota transplantation as a treatment for ulcerative colitis?
- Clostridioides Infection in Patients with Inflammatory Bowel Disease
- Next Generation Fecal Microbiota Transplantation
- Diagnostic Stewardship on the Extra-Analytical Phase to Secure Quality Assurance of Diagnostic Tests for Clostridioides difficile Infection