Infect Chemother.
2016 Sep;48(3):151-159. 10.3947/ic.2016.48.3.151.
Antibiotic Control Policies in South Korea, 2000-2013
- Affiliations
-
- 1Department of Internal Medicine, Inje University Sanggye-Paik Hospital, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. mdohmd@snu.ac.kr
- KMID: 2466426
- DOI: http://doi.org/10.3947/ic.2016.48.3.151
Abstract
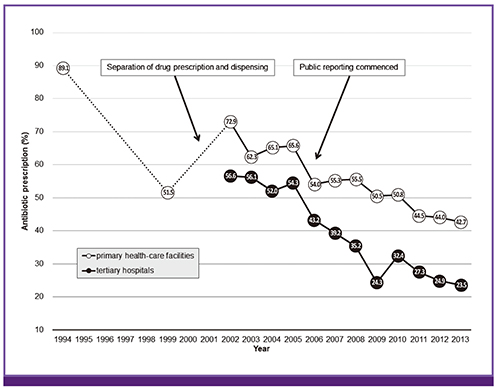
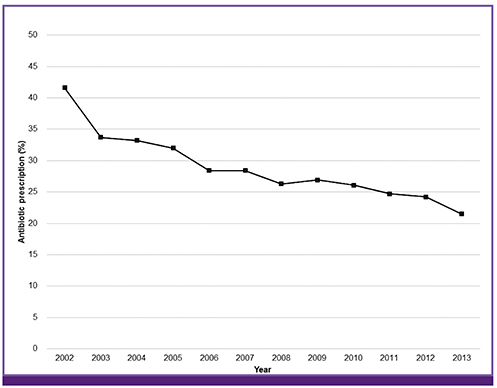
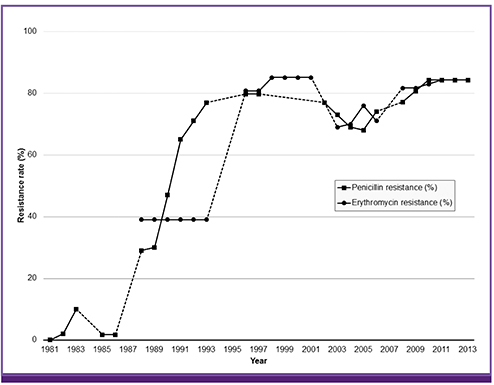
- Antibiotic stewardship is a key strategy for limiting antibiotic resistance. Over the last decade the South Korean government has implemented a series of healthcare policies directed to this end, consisting of legislative separation of drug prescribing and dispensing, antibiotic utilization reviews, healthcare quality assessment, and public reporting. As a result, the proportion of antibiotic prescriptions for acute upper respiratory tract infections in primary healthcare facilities decreased from 72.9% in 2002 to 42.7% in 2013. However, no significant decrease in antibiotic resistance occurred over the same period in clinically important bacteria such as Streptococcus pneumoniae. These government-driven policies played a pivotal role in improving antibiotic use for outpatients and surgical patients in South Korea. However, to achieve long-lasting successful outcomes, coordinated efforts and communications among the stakeholders, including physicians and medical societies, are needed.
Keyword
MeSH Terms
-
Bacteria
Delivery of Health Care
Drug Prescriptions
Drug Resistance, Microbial
Drug Utilization Review
Health Policy
Humans
Inappropriate Prescribing
Korea*
Outpatients
Prescriptions
Primary Health Care
Quality Assurance, Health Care
Respiratory Tract Infections
Societies, Medical
Streptococcus pneumoniae
Utilization Review
Figure
Cited by 2 articles
-
Development of Antibiotic Classification for Measuring Antibiotic Usage in Korean Hospitals Using a Modified Delphi Method
Bongyoung Kim, Young Kyung Yoon, Dong-Sook Kim, Su Jin Jeong, Song Vogue Ahn, Sun Hee Park, Ki Tae Kwon, Hong Bin Kim, Yoon Soo Park, Shin-Woo Kim, Sungmin Kiem, Jun Yong Choi, , ,
J Korean Med Sci. 2020;35(30):e241. doi: 10.3346/jkms.2020.35.e241.Can the Use of Antibiotics Alter the Susceptibility to Allergic Diseases?
So-Yeon Lee
Allergy Asthma Immunol Res. 2018;10(5):425-427. doi: 10.4168/aair.2018.10.5.425.
Reference
-
1. World Health Organization (WHO). Antimicrobial resistance: global report on surveillance, 2014. Accessed 10 November 2014. Available at: http://www.who.int/drugresistance/documents/surveillancereport/en/.2. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006; 42:Suppl 2. S82–9.
Article3. World Health Organization (WHO). World Health Day 2011. Combat drug resistance: no action today means no cure tomorrow. Accessed 10 November 2014. Available at: http://www.who.int/mediacentre/news/statements/2011/whd_20110407/en/.4. MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005; 18:638–656.
Article5. Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, Ramsay CR, Wiffen PJ, Wilcox M. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013; 4:CD003543.
Article6. Huttner B, Harbarth S, Nathwani D; ESCMID Study Group for Antibiotic Policies (ESGAP). Success stories of implementation of antimicrobial stewardship: a narrative review. Clin Microbiol Infect. 2014; 20:954–962.
Article7. Goossens H, Coenen S, Costers M, De Corte S, De Sutter A, Gordts B, Laurier L, Struelens M. Achievements of the Belgian Antibiotic Policy Coordination Committee (BAPCOC). Euro Surveill. 2008; 13:19036.
Article8. Xiao Y, Zhang J, Zheng B, Zhao L, Li S, Li L. Changes in Chinese policies to promote the rational use of antibiotics. PLoS Med. 2013; 10:e1001556.
Article9. Sabuncu E, David J, Bernède-Bauduin C, Pépin S, Leroy M, Boëlle PY, Watier L, Guillemot D. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007. PLoS Med. 2009; 6:e1000084.
Article10. Chahwakilian P, Huttner B, Schlemmer B, Harbarth S. Impact of the French campaign to reduce inappropriate ambulatory antibiotic use on the prescription and consultation rates for respiratory tract infections. J Antimicrob Chemother. 2011; 66:2872–2879.
Article11. Park S, Moon OR. Patterns of antibiotics utilization in some respiratory diseases in clinics. J Korean Soc Qual Assur Health Care. 1998; 5:58–75.12. Uh GS, Byeon JJ, Shin HC, Lee JH, Choi YS, Roh YK. Antibiotics prescription pattern of family practitioners for respiratory tract infections. Korean J Fam Med. 2000; 21:901–913.13. Mangione-Smith R, Wong L, Elliott MN, McDonald L, Roski J. Measuring the quality of antibiotic prescribing for upper respiratory infections and bronchitis in 5 US health plans. Arch Pediatr Adolesc Med. 2005; 159:751–757.
Article14. Murphy M, Bradley CP, Byrne S. Antibiotic prescribing in primary care, adherence to guidelines and unnecessary prescribing--an Irish perspective. BMC Fam Pract. 2012; 13:43.15. Panasiuk L, Lukas W, Paprzycki P, Verheij T, Godycki-Ćwirko M, Chlabicz S. Antibiotics in the treatment of upper respiratory tract infections in Poland. Is there any improvement? J Clin Pharm Ther. 2010; 35:665–669.
Article16. Nadeem Ahmed M, Muyot MM, Begum S, Smith P, Little C, Windemuller FJ. Antibiotic prescription pattern for viral respiratory illness in emergency room and ambulatory care settings. Clin Pediatr (Phila). 2010; 49:542–547.
Article17. Yun JM, Shin DW, Hwang SS, Cho J, Nam YS, Kim JH, Cho BL. Effect of public disclosure on antibiotic prescription rate for upper respiratory tract infections. JAMA Intern Med. 2015; 175:445–447.
Article18. Kim ES, Park SW, Lee CS, Kwak YG, Moon C, Kim BN. Impact of a national hospital evaluation program using clinical performance indicators on the use of surgical antibiotic prophylaxis in Korea. Int J Infect Dis. 2012; 16:e187–92.
Article19. Chun CB, Kim SY, Lee JY, Lee SY. Republic of Korea: Health system review. Health Syst Transit. 2009; 11:1–184.20. Health Insurance Review and Assessment Service. Comprehensive quality report of National Health Insurance 2010. Seoul: Health Insurance Review and Assessment Service;2011.21. Health Insurance Review & Assessment Service. Results of quality asessment of prescriptions in 2013. Accessed 4 November 2014. Available at: http://www.hira.or.kr/ebook/bbd89385-c44d-45ab-acf4-fa5f802d0412/140912_Page_img/extra/140912.pdf.22. Lee EK, Bae JM, Park KH, Park BJ, Lee JY, Jang SM, Lee YH, Park KJ. Drug use evaluation. Seoul, Republic of Korea: Korea Institute for Health and Social Affairs;2000.23. Kwon S. Pharmaceutical reform and physician strikes in Korea: separation of drug prescribing and dispensing. Soc Sci Med. 2003; 57:529–538.
Article24. Kim HJ, Chung W, Lee SG. Lessons from Korea’s pharmaceutical policy reform: the separation of medical institutions and pharmacies for outpatient care. Health Policy. 2004; 68:267–275.
Article25. Kang HY, Kim SJ, Cho W, Lee S. Consumer use of publicly released hospital performance information: assessment of the National Hospital Evaluation Program in Korea. Health Policy. 2009; 89:174–183.
Article26. Health Insurance Review & Assessment Service. Results of quality asessment of prescriptions in 2012. Accessed 4 July 2013. Available at: http://www.hira.or.kr/cms/notice/01/__icsFiles/afieldfile/2013/07/04/2012_report.pdf.27. Health Insurance Review & Assessment Service. Report of drug utilization review in the latter half of 2013. Accessed 11 November 2014. Available at: http://www.hira.or.kr/dummy.do?pgmid=HIRAA020045040000&cmsurl=/cms/open/04/02/03/04/1325117_25309.html&subject=2013%eb%85%84+%ed%95%98%eb%b0%98%ea%b8%b0+%ec%95%bd%ec%a0%9c%ea%b8%89%ec%97%ac%ec%a0%81%ec%a0%95%ec%84%b1+%ed%8f%89%ea%b0%80%ea%b2%b0%ea%b3%bc+%eb%b3%b4%ea%b3%a0%ec%84%9c#none.28. Lee YS, Kim MK, Kim YI, Shin YS, Lee HJ, Ahn HS. Private practitioners’ antimicrobial prescription patterns for acute respiratory infections in children. J Korean Public Health Assoc. 1991; 17:3–19.29. Kim NS, Jang SN, Jang SM. Factors influencing antibiotics prescribing of primary health physicians in acute upper respiratory infections. J Prev Med Public Health. 2005; 38:1–8.30. Kim DS. Physician’s belief on antibiotic use in upper respiratory tract infections. HIRA Policy Trend. 2011; 5:33–41.31. Cho HJ, Woo SK, Hong CT, Suh EK. Comparison of prescription behaviors between practicing physicians and pharmacists by simulated patients with common cold. J Korean Acad Fam Med. 2001; 22:1394–1399.32. Cho HJ, Kim CB. Prescription behaviours of office-based doctors to standardized common cold patients in Korea. Pharmacoepidemiol Drug Saf. 2002; 11:401–405.
Article33. Cho HJ, Kim CB. Discolored nasal discharge did not increase the antibiotic prescription rate for the common cold patients. Pharmacoepidemiol Drug Saf. 2005; 14:139–141.
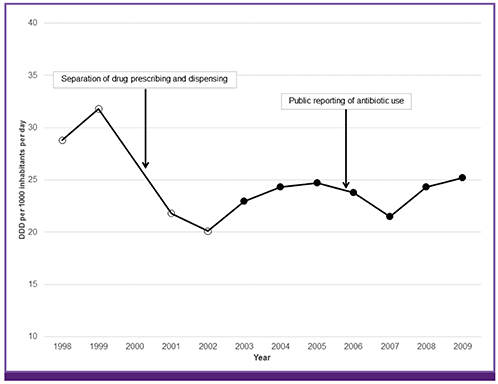
Article34. Chung HJ, Lee HJ, Kim ES, Lee JS, Chung MH. Antibiotic prescription by primary care physicians for upper respiratory infections. Infect Chemother. 2007; 39:125–132.35. Lee EK. Study on the relationship of antibiotic use and resistance. Seoul: Korea Food & Drug Administration;2002.36. Health Insurance Review and Assessment Service. Analysis and evaluation of antibiotic use. Seoul: Health Insurance Review and Assessment Service;2006.37. Lee YS, Kwon JW, Oh OH, Sohn HS. Temporal decrease in overall antibiotic consumption accompanying antibiotic prescribing rate disclosure policy: evidence from analysis of national health insurance claims data in South Korea. Arch Pharm Res. 2014; 37:1295–1300.
Article38. Organisation for Economic Co-operation and Development. OECD Health Statistics 2014. Accessed 11 November 2014. Available at: http://www.oecd.org/els/health-systems/health-data.htm.39. SNUHMC Institute of Health Policy & Management. Outcome evaluation models for the quality assessment of medical services. Accessed 31 July 2012. Available at: http://www.snu-dhpm.ac.kr/pds/article.html?code=report1&number=1703&keyfield=&key=.40. Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, Lu M, So TM, Hsueh PR, Yasin RM, Carlos CC, Pham HV, Lalitha MK, Shimono N, Perera J, Shibl AM, Baek JY, Kang CI, Ko KS, Peck KR; ANSORP Study Group. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012; 56:1418–1426.
Article41. Hong SI, Kwon TH, Park CS, Suck JS, Kim SI. Analysis of antimicrobial susceptibility patterns of various microorganisms isolated from Seoul National University Hospital: statistical analysis on various clinical isolates during resecent 4 years (1980-1983). Korean J Clin Pathol. 1984; 4:149–162.42. Lee SY, Chong Y. Prevalence of penicillin-resistant Streptococcus pneumoniae and antimicrobial susceptibility of beta-hemolytic Streptococcus and Enterococcus. J Korean Soc Chemother. 1986; 4:44–51.43. Lee H, Kim CK, Lee J, Lee SH, Ahn JY, Hong SG, Park YJ, Jeong SH, Kim EC, Lee WK, Uh Y, Shin JH, Choi TY, Kwak HS, Lee K. Antimicrobial resistance of clinically important bacteria isolated from 12 hospitals in Korea in 2005 and 2006. Korean J Clin Microbiol. 2007; 10:59–69.44. Chong Y, Lee K, Kwon OH, Henrichsen J. Capsular types and antimicrobial resistance of Streptococcus pneumoniae isolated in Korea. Eur J Clin Microbiol Infect Dis. 1995; 14:528–531.
Article45. Song JH, Lee NY, Ichiyama S, Yoshida R, Hirakata Y, Fu W, Chongthaleong A, Aswapokee N, Chiu CH, Lalitha MK, Thomas K, Perera J, Yee TT, Jamal F, Warsa UC, Vinh BX, Jacobs MR, Appelbaum PC, Pai CH. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian network for surveillance of resistant pathogens (ANSORP) study. Clin Infect Dis. 1999; 28:1206–1211.
Article46. Song JH, Chang HH, Suh JY, Ko KS, Jung SI, Oh WS, Peck KR, Lee NY, Yang Y, Chongthaleong A, Aswapokee N, Chiu CH, Lalitha MK, Perera J, Yee TT, Kumararasinghe G, Jamal F, Kamarulazaman A, Parasakthi N, Van PH, So T, Ng TK; ANSORP Study Group. Macrolide resistance and genotypic characterization of Streptococcus pneumoniae in Asian countries: a study of the Asian Network for Surveillance of Resistant Pathogens (ANSORP). J Antimicrob Chemother. 2004; 53:457–463.
Article47. Bae SM, Lee SK. Prevalence of serotype and multidrug resistance of Streptococcus pneumoniae isolated from patients with community-acquired pneumonia. Public Health Wkly Rep. 2014; 7:573–578.48. Lee H, Choi EH, Lee HJ. Efficacy and effectiveness of extended-valency pneumococcal conjugate vaccines. Korean J Pediatr. 2014; 57:55–66.
Article49. Song JY, Cheong HJ. Pneumococcal vaccine. J Korean Med Assoc. 2014; 57:780–788.
Article50. Song JY, Cheong HJ, Heo JY, Noh JY, Seo YB, Kim IS, Choi WS, Kim WJ. Outpatient-based pneumococcal vaccine campaign and survey of perceptions about pneumococcal vaccination in patients and doctors. Yonsei Med J. 2013; 54:469–475.
Article51. Huttner B, Goossens H, Verheij T, Harbarth S; CHAMP consortium. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010; 10:17–31.
Article52. Fung CH, Lim YW, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med. 2008; 148:111–123.
Article53. Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care--reasons for caution. N Engl J Med. 2014; 370:1673–1676.
Article54. Haustein T, Gastmeier P, Holmes A, Lucet JC, Shannon RP, Pittet D, Harbarth S. Use of benchmarking and public reporting for infection control in four high-income countries. Lancet Infect Dis. 2011; 11:471–481.
Article55. Thompson PL, Gilbert RE, Long PF, Saxena S, Sharland M, Wong IC. Has UK guidance affected general practitioner antibiotic prescribing for otitis media in children? J Public Health (Oxf). 2008; 30:479–486.
Article56. Oh OH. Study on total volume of systemic antibacterials used of human body (2008-2009). Soeul: KCDC;2010.57. Chow AW, Benninger MS, Brook I, Brozek JL, Goldstein EJ, Hicks LA, Pankey GA, Seleznick M, Volturo G, Wald ER, File TM Jr; Infectious Diseases Society of America. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012; 54:e72–112.
Article58. DeMuri GP, Wald ER. Clinical practice. Acute bacterial sinusitis in children. N Engl J Med. 2012; 367:1128–1134.59. Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, Martin JM, Van Beneden C. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012; 55:1279–1282.
Article60. Mölstad S, Erntell M, Hanberger H, Melander E, Norman C, Skoog G, Lundborg CS, Söderström A, Torell E, Cars O. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish Strama programme. Lancet Infect Dis. 2008; 8:125–132.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antibiotic Resistance of
Acinetobacter baumannii in Iran: A Systemic Review of the Published Literature - Current status of tobacco use, cessation and control policy in Korea
- Potentially Inappropriate Prescriptions of Antibiotics in Patients with Acute Rhinosinusitis in Ambulatory Settings in South Korea
- A Comparison of Medical Education Policies in Japan and Singapore with a Focus on Governance: Implications for Korea
- Evaluation of the tobacco control policies of the Moon Jae-in government
- Antibiotic Resistance of