J Bone Metab.
2019 Nov;26(4):271-277. 10.11005/jbm.2019.26.4.271.
Use of Bone Turnover Markers in Clinical Practice for the Management of Osteoporosis in Korea: From the Survey on the Prescription Pattern of Bone Turnover Markers
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Inha University Hospital, Inha University School of Medicine, Incheon, Korea. sbhongmd@inha.ac.kr
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Kyung Hee University Hospital, Seoul, Korea.
- 3Department of Orthopaedic Surgery, Gyeongsang National University Hospital, Jinju, Korea.
- 4Department of Obstetrics and Gynecology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Pusan National University Hospital, Busan, Korea.
- 6Department of Orthopaedic Surgery, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 7Division of Endocrinology, Department of Internal Medicine, Wonkwang University Sanbon Hospital, Wonkwang University School of Medicine, Gunpo, Korea.
- 8Division of Endocrinology and Metabolism, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 9Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2465029
- DOI: http://doi.org/10.11005/jbm.2019.26.4.271
Abstract
- BACKGROUND
There has been interest in the clinical potential of bone turnover markers (BTMs) as tools both for assessing fracture risk and for monitoring treatment. However, the practical use of BTMs has been limited by their biological variability and difficulties in the interpretation of results. We investigated the current situation of application of BTMs by clinicians in Korea for the management of osteoporosis through a survey asking the patterns of BTMs prescription in clinical practice.
METHODS
The survey was conducted online using the "google survey" by the BTM committee authorized by the Korean Society for Bone and Mineral Research.
RESULTS
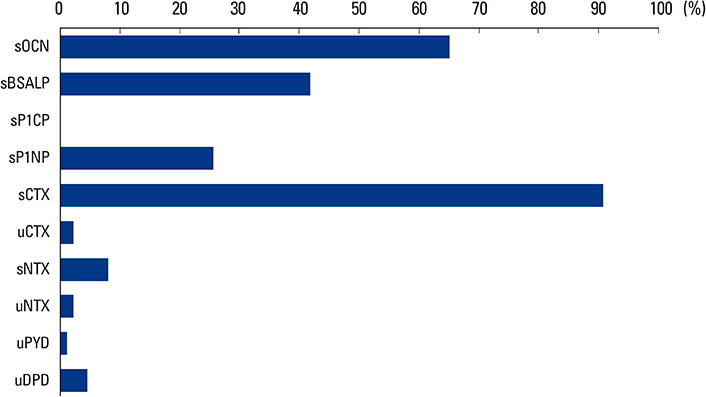
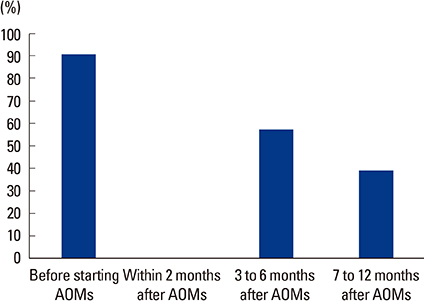
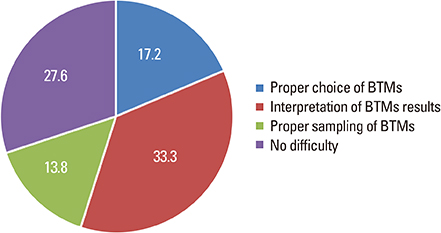
Total 108 clinicians responded the survey. Most of the respondents prescribed BTMs (80.6%) when they prescribed anti-osteoporotic medications (AOMs). The most frequently prescribed bone resorption and formation markers were serum C-terminal telopeptide of type I collagen (90.7%) and osteocalcin (65.1%), respectively. BTMs were mostly prescribed before starting AOMs (90.8%) and used for the purpose of evaluating treatment response (74.4%). Treatment response and compliance to AOMs were evaluated according to the change of absolute value of BTMs (55.1%). The respondents complained difficulties in the interpretation of BTMs (33.3%), the choice of proper BTMs (17.2%), and the proper sample preparation and handling (13.8%).
CONCLUSIONS
In Korea, most of clinicians recognized the benefit of BTMs in the management of osteoporosis. However, there are limitations in the broad use of these markers in clinical practice. Therefore, a clear recommendation for BTM in Korea enhances their use in clinical practice.
Keyword
MeSH Terms
Figure
Reference
-
1. Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007; 22:465–475.
Article2. Kang HY, Yang KH, Kim YN, et al. Incidence and mortality of hip fracture among the elderly population in South Korea: a population-based study using the national health insurance claims data. BMC Public Health. 2010; 10:230.
Article3. Kanis JA, McCloskey EV, Johansson H, et al. A reference standard for the description of osteoporosis. Bone. 2008; 42:467–475.
Article4. Kanis JA, Melton LJ 3rd, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994; 9:1137–1141.
Article5. Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996; 11:1531–1538.
Article6. Hans D, Goertzen AL, Krieg MA, et al. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011; 26:2762–2769.
Article7. Jain RK, Vokes T. Dual-energy X-ray absorptiometry. J Clin Densitom. 2017; 20:291–303.
Article8. Brown JP, Albert C, Nassar BA, et al. Bone turnover markers in the management of postmenopausal osteoporosis. Clin Biochem. 2009; 42:929–942.
Article9. Szulc P, Delmas PD. Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int. 2008; 19:1683–1704.
Article10. Vasikaran SD. Utility of biochemical markers of bone turnover and bone mineral density in management of osteoporosis. Crit Rev Clin Lab Sci. 2008; 45:221–258.
Article11. Bergmann P, Body JJ, Boonen S, et al. Evidence-based guidelines for the use of biochemical markers of bone turnover in the selection and monitoring of bisphosphonate treatment in osteoporosis: a consensus document of the Belgian Bone Club. Int J Clin Pract. 2009; 63:19–26.
Article12. Hannon R, Eastell R. Preanalytical variability of biochemical markers of bone turnover. Osteoporos Int. 2000; 11 Suppl 6:S30–S44.
Article13. Seibel MJ, Lang M, Geilenkeuser WJ. Interlaboratory variation of biochemical markers of bone turnover. Clin Chem. 2001; 47:1443–1450.
Article14. Vasikaran S, Eastell R, Bruyère O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011; 22:391–420.
Article15. Park SY, Ahn SH, Yoo JI, et al. Clinical application of bone turnover markers in osteoporosis in Korea. J Bone Metab. 2019; 26:19–24.
Article16. Nishizawa Y, Miura M, Ichimura S, et al. Executive summary of the Japan osteoporosis society guide for the use of bone turnover markers in the diagnosis and treatment of osteoporosis (2018 Edition). Clin Chim Acta. 2019; 498:101–107.
Article17. Szulc P, Naylor K, Hoyle NR, et al. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. 2017; 28:2541–2556.
Article18. Bhattoa HP. Laboratory aspects and clinical utility of bone turnover markers. Ejifcc. 2018; 29:117–128.19. Bauer DC, Garnero P, Hochberg MC, et al. Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res. 2006; 21:292–299.
Article20. Eastell R, Barton I, Hannon RA, et al. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003; 18:1051–1056.
Article21. Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004; 89:1117–1123.
Article22. Delmas PD, Vrijens B, Eastell R, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007; 92:1296–1304.
Article23. Christiansen C, Riis BJ, Rødbro P. Prediction of rapid bone loss in postmenopausal women. Lancet. 1987; 1:1105–1108.
Article24. Garnero P, Sornay-Rendu E, Chapuy MC, et al. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996; 11:337–349.
Article25. Bauer D, Krege J, Lane N, et al. National bone health alliance bone turnover marker project: Current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos Int. 2012; 23:2425–2433.
Article26. Weiss TW, Siris ES, Barrett-Connor E, et al. Osteoporosis practice patterns in 2006 among primary care physicians participating in the NORA study. Osteoporos Int. 2007; 18:1473–1480.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of Biochemical Markers of Bone turnover in Pre-, Peri-and Postmenopausal Women
- Biochemical Markers of Bone Turnover
- Study for Usefulness of Total Alkaline Phosphatase as a Biochemical Markers of Bone Turnover in Healthy Menopausal Women
- Bone Mineral Density and Bone Turnover Markers after One-year Teriparatide Treatment in Korean Postmenopausal Women with Osteoporosis
- Changes of markers of bone turnover and spinal BMD after 1 year treatment according to treatment strategies & predictability of changes of BMD by changes of bone markers in Korean postmenopausal women with osteoporosis