Cancer Res Treat.
2019 Apr;51(2):758-768. 10.4143/crt.2018.421.
Application of the International Metastatic Renal Cell Carcinoma Database Consortium and Memorial Sloan Kettering Cancer Center Risk Models in Patients with Metastatic Non-Clear Cell Renal Cell Carcinoma: A Multi-Institutional Retrospective Study Using the Korean Metastatic Renal Cell Carcinoma Registry
- Affiliations
-
- 1Department of Urology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 2Department of Urology, Center for Prostate Cancer, National Cancer Center, Goyang, Korea. cjs5225@ncc.re.kr
- 3Biometric Research Branch, Center for Prostate Cancer, National Cancer Center, Goyang, Korea.
- 4Department of Urology, Sungkyunkwan University College of Medicine, Seoul, Korea.
- 5Department of Urology, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Urology, University of Ulsan College of Medicine, Seoul, Korea.
- 7Department of Urology, Medical School, Chonnam National University, Hwasun, Korea.
- 8Department of Urology, Wonkwang University School of Medicine and Hospital, Iksan, Korea.
- 9Department of Urology, Seoul St. Mary's Hospital, The Catholic University, Seoul, Korea.
- 10Department of Urology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
- KMID: 2464421
- DOI: http://doi.org/10.4143/crt.2018.421
Abstract
- PURPOSE
The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) and the Memorial Sloan Kettering Cancer Center (MSKCC) risk models were developed predominantly with clear cell renal cell carcinoma (RCC). Accordingly, whether these two models could be applied to metastatic non-clear cell RCC (mNCCRCC) as well has not been well-known and was investigated herein.
MATERIALS AND METHODS
From the Korean metastatic RCC registry, a total of 156 patients (8.1%) with mNCCRCC among the entire cohort of 1,922 patients were analyzed. Both models were applied to predict first-line progression-free survival (PFS), total PFS, and cancer-specific survival (CSS).
RESULTS
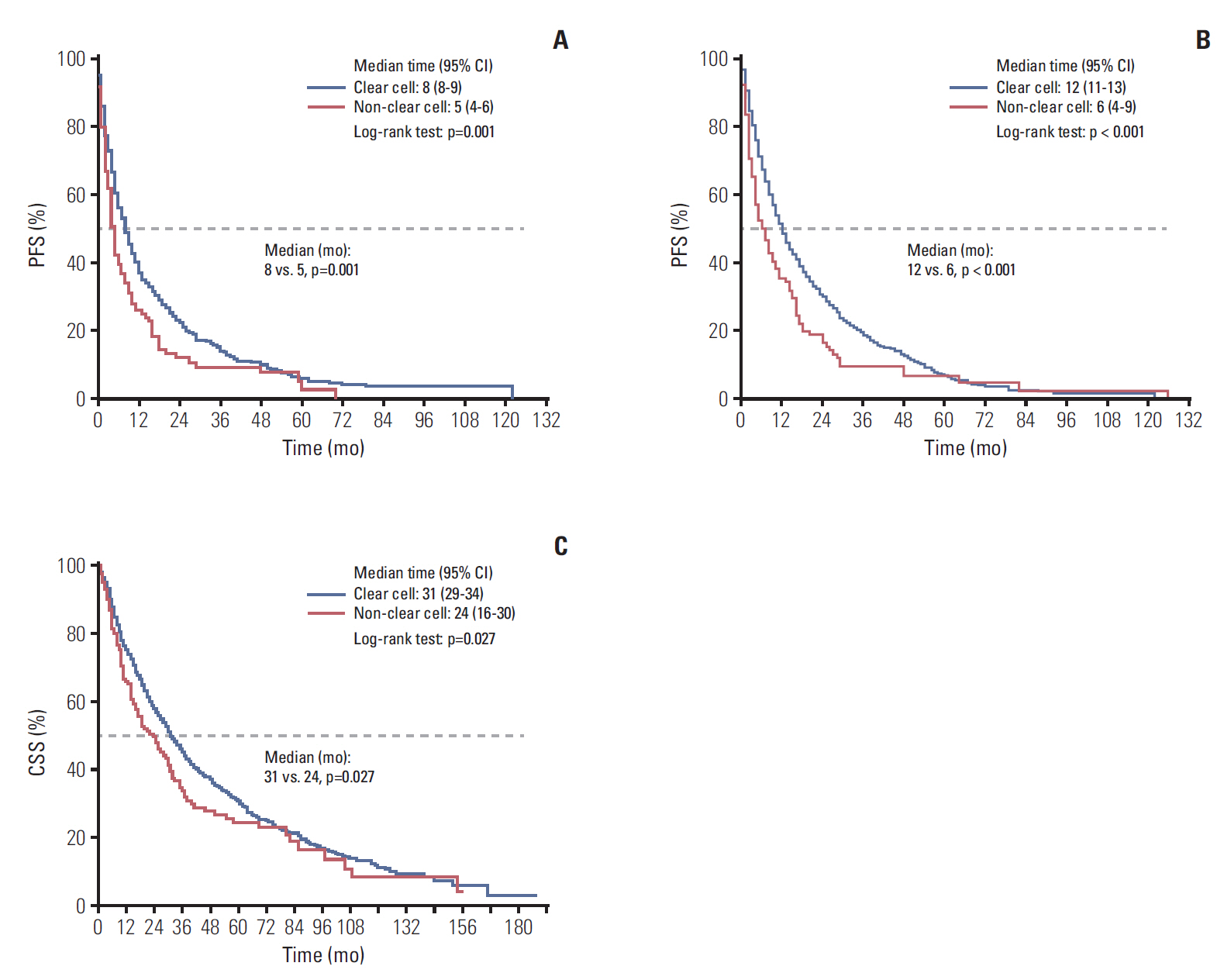
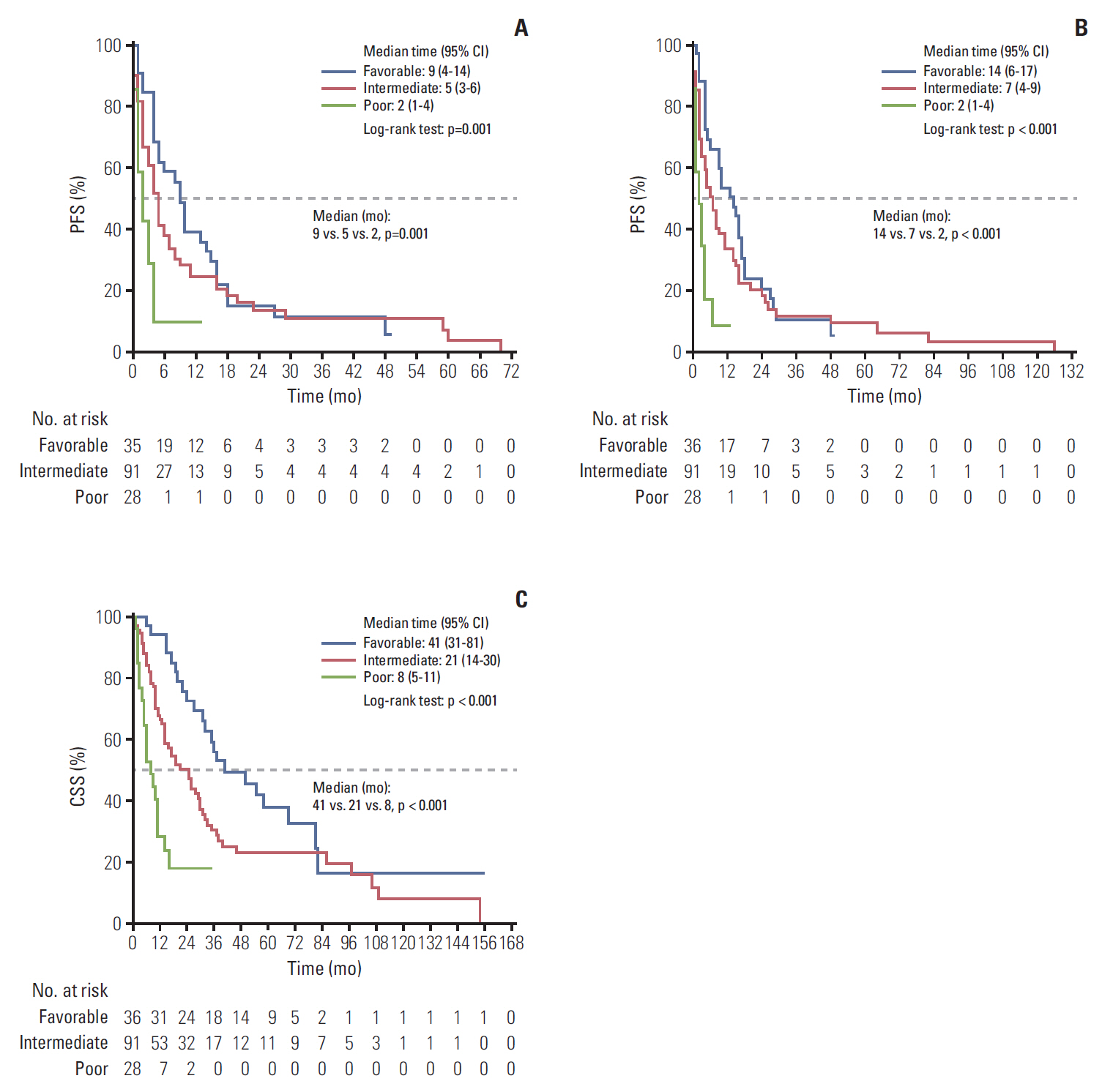
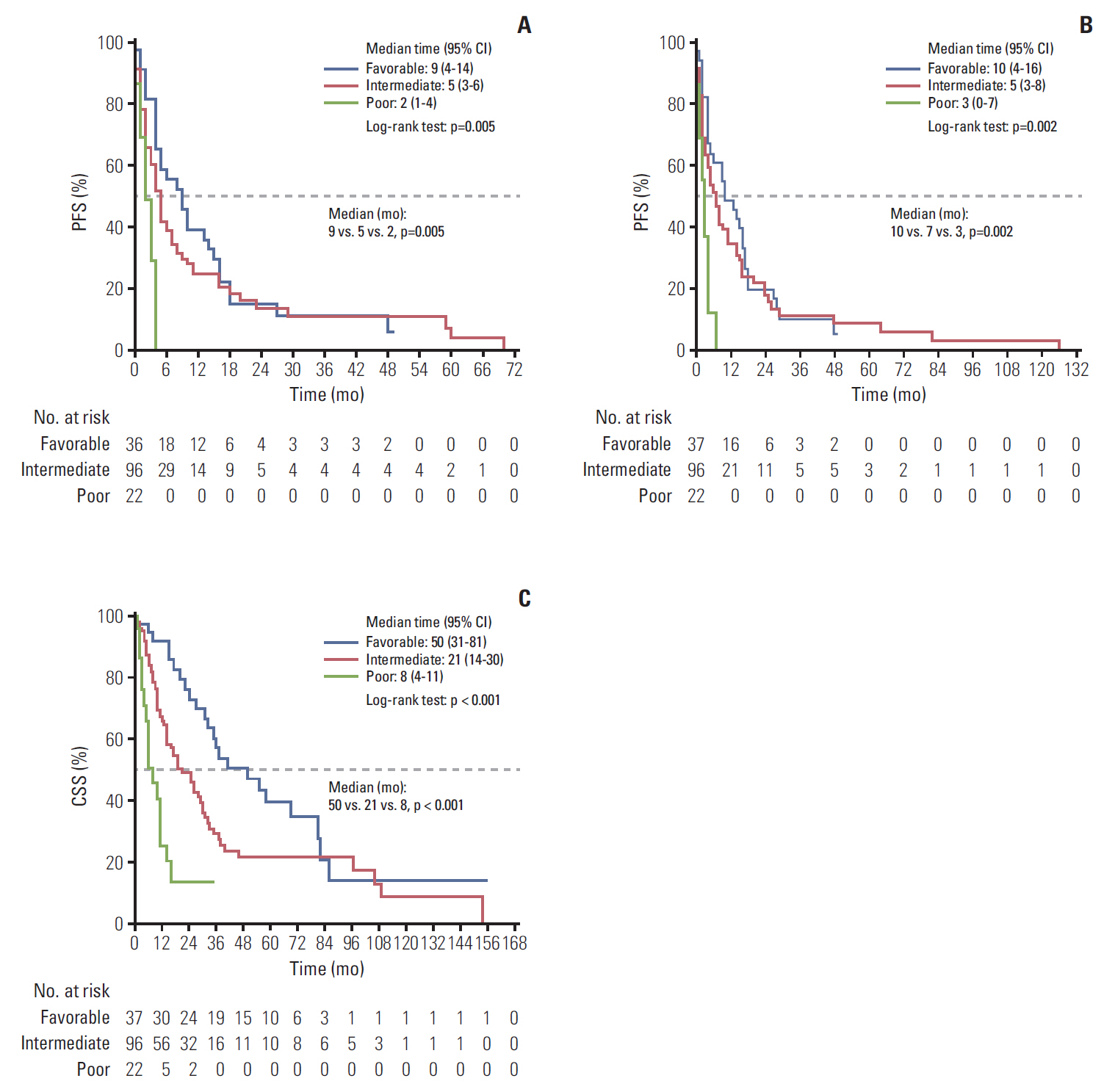
The median first-line PFS, total PFS, and CSS were 5, 6, and 24 months, respectively. The IMDC risk model reliably discriminated three risk groups to predict survival: the median first-line PFS, total PFS, and CSS for the favorable, intermediate, and poor risk groups were 9, 5, and, 2 months (p=0.001); 14, 7, and 2 months (p < 0.001); and 41, 21, and 8 months (p < 0.001), all respectively. The MSKCC risk model also reliably differentiated three risk groups: 9, 5, and, 2 months (p=0.005); 10, 7, and 3 months (p=0.002); and 50, 21, and 8 months (p < 0.001), also all respectively. The concordance indices were 0.632 with the IMDC model and 0.643 with the MSKCC model for first-line PFS: 0.748 and 0.655 for CSS.
CONCLUSION
The current IMDC and MSKCC risk models reliably predict first-line PFS, total PFS, and CSS in mNCCRCC.
MeSH Terms
Figure
Reference
-
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.
Article2. Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006; 49:798–805.
Article3. Motzer RJ, Agarwal N, Beard C, Bolger GB, Boston B, Carducci MA, et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw. 2009; 7:618–30.4. Oudard S, George D, Medioni J, Motzer R. Treatment options in renal cell carcinoma: past, present and future. Ann Oncol. 2007; 18 Suppl 10:x25–31.
Article5. Patard JJ, Pignot G, Escudier B, Eisen T, Bex A, Sternberg C, et al. ICUD-EAU International Consultation on Kidney Cancer 2010: treatment of metastatic disease. Eur Urol. 2011; 60:684–90.
Article6. Atkins MB, Plimack ER, Puzanov I, Fishman MN, McDermott DF, Cho DC, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018; 19:405–15.
Article7. Powles T, Albiges L, Staehler M, Bensalah K, Dabestani S, Giles RH, et al. Updated European Association of Urology guidelines recommendations for the treatment of first-line metastatic clear cell renal cancer. Eur Urol. 2018; 73:311–5.
Article8. Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009; 27:5794–9.
Article9. Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013; 14:141–8.
Article10. Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004; 22:454–63.
Article11. Kroeger N, Xie W, Lee JL, Bjarnason GA, Knox JJ, Mackenzie MJ, et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer. 2013; 119:2999–3006.
Article12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article13. Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004; 23:2109–23.14. Fernandez-Pello S, Hofmann F, Tahbaz R, Marconi L, Lam TB, Albiges L, et al. A systematic review and meta-analysis comparing the effectiveness and adverse effects of different systemic treatments for non-clear cell renal cell carcinoma. Eur Urol. 2017; 71:426–36.15. Armstrong AJ, Halabi S, Eisen T, Broderick S, Stadler WM, Jones RJ, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016; 17:378–88.
Article16. Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005; 353:2477–90.
Article17. Joung JY, Lim J, Oh CM, Jung KW, Cho H, Kim SH, et al. Current trends in the incidence and survival rate of urological cancers in Korea. Cancer Res Treat. 2017; 49:607–15.
Article18. Upton MP, Parker RA, Youmans A, McDermott DF, Atkins MB. Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother. 2005; 28:488–95.
Article19. Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI, Knox JJ, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. 2015; 16:293–300.
Article20. Kwon WA, Cho IC, Yu A, Nam BH, Joung JY, Seo HK, et al. Validation of the MSKCC and Heng risk criteria models for predicting survival in patients with metastatic renal cell carcinoma treated with sunitinib. Ann Surg Oncol. 2013; 20:4397–404.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Metastatic Renal Cell Carcinoma to the Gallbladder
- A Case of Renal Cell Carcinoma Metastatic to the Right Zygoma
- A Case of Renal Cell Carcinoma Metastatic to the Scalp
- Metastatic Clear Cell Renal Cell Carcinoma to the Thyroid Gland
- Perineal Metastatic Clear Cell Renal Cell Carcinoma: A Case Report