J Korean Neurosurg Soc.
2019 Nov;62(6):712-722. 10.3340/jkns.2018.0226.
Factors Related to Successful Energy Transmission of Focused Ultrasound through a Skull: A Study in Human Cadavers and Its Comparison with Clinical Experiences
- Affiliations
-
- 1Department of Neurosurgery, Brain Research Institute, Yonsei University College of Medicine, Seoul, Korea. JCHANG@yuhs.ac
- 2InSightec Ltd., Tirat Carmel, Israel.
- KMID: 2463663
- DOI: http://doi.org/10.3340/jkns.2018.0226
Abstract
OBJECTIVE
Although magnetic resonance guided focused ultrasound (MRgFUS) has been used as minimally invasive and effective neurosurgical treatment, it exhibits some limitations, mainly related to acoustic properties of the skull barrier. This study was undertaken to identify skull characteristics that contribute to optimal ultrasonic energy transmission for MRgFUS procedures.
METHODS
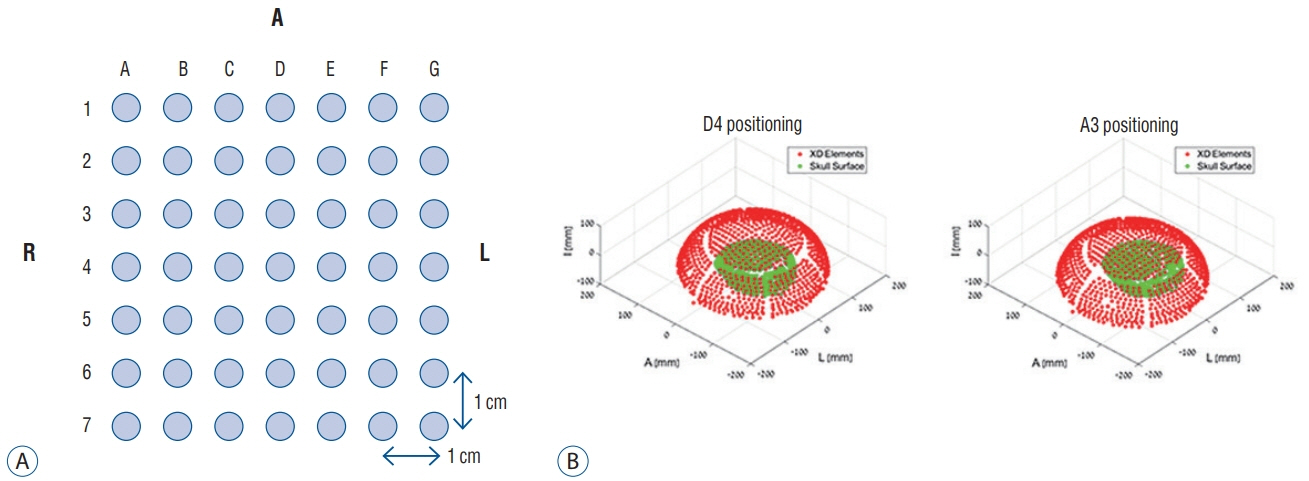
For ex vivo skull experiments, various acoustic fields were measured under different conditions, using five non-embalmed cadaver skulls. For clinical skull analyses, brain computed tomography data of 46 patients who underwent MRgFUS ablations (18 unilateral thalamotomy, nine unilateral pallidotomy, and 19 bilateral capsulotomy) were retrospectively reviewed. Patients' skull factors and sonication parameters were comparatively analyzed with respect to the cadaveric skulls.
RESULTS
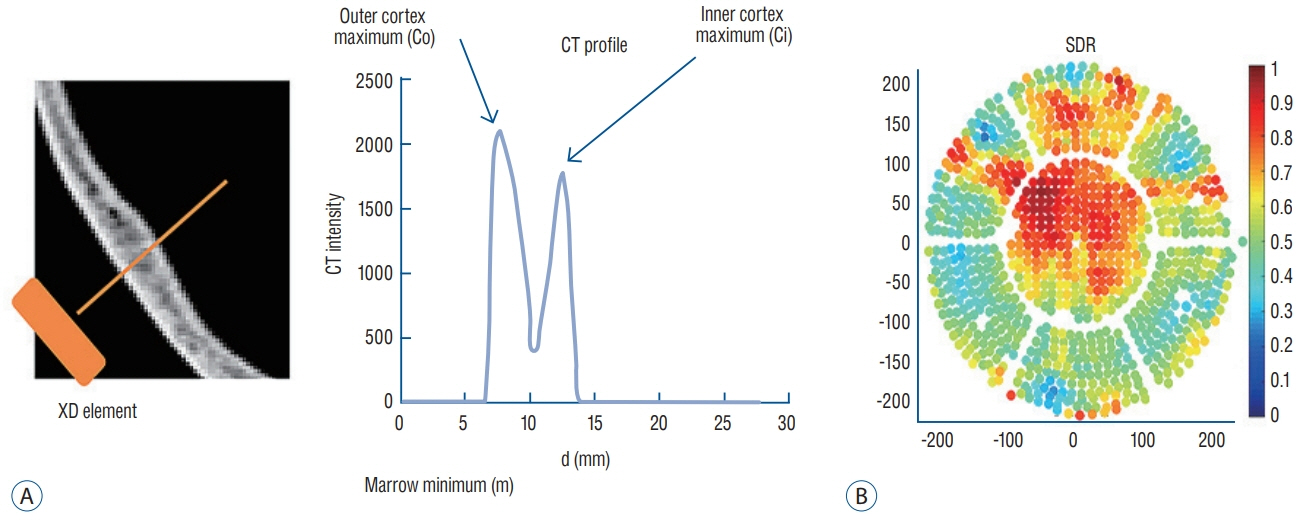
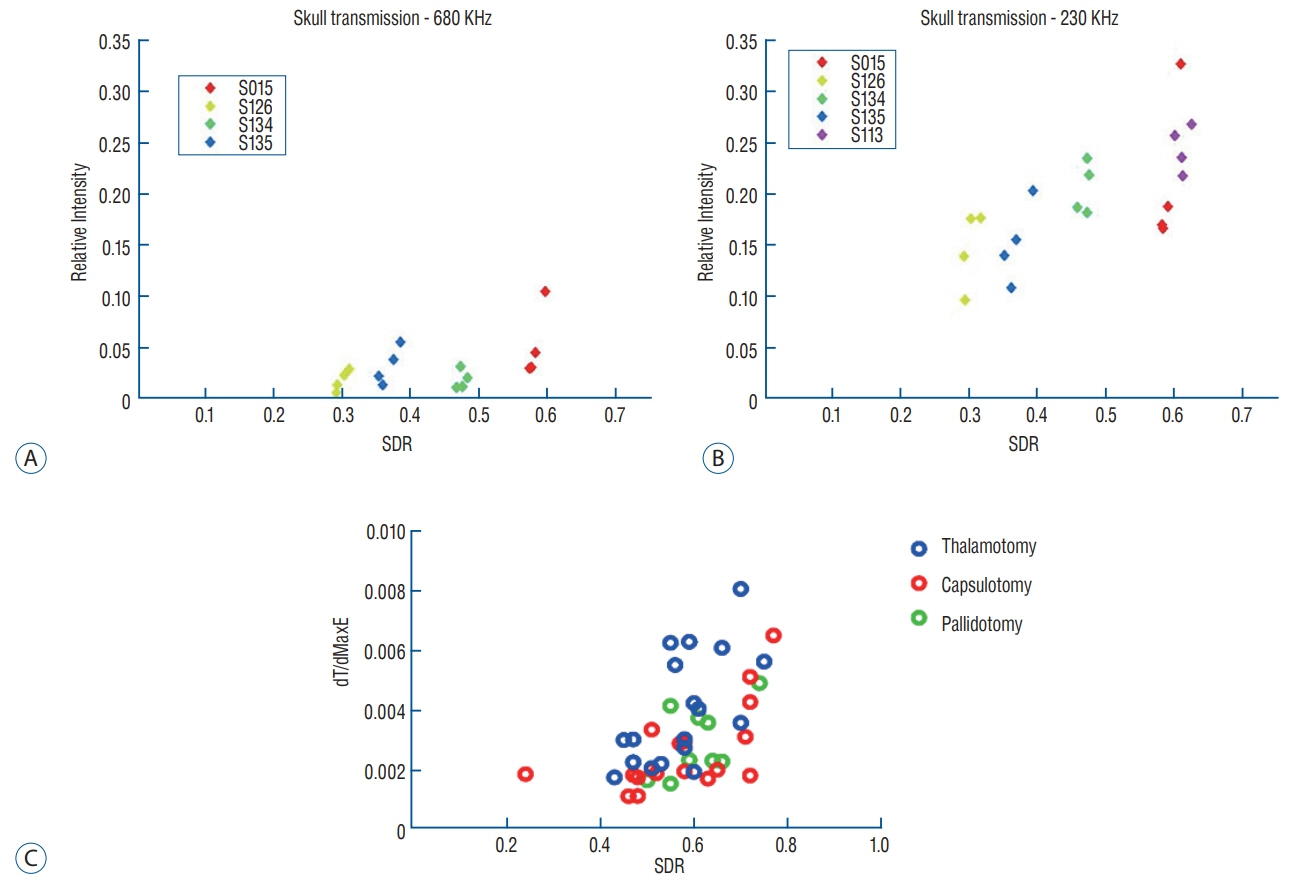
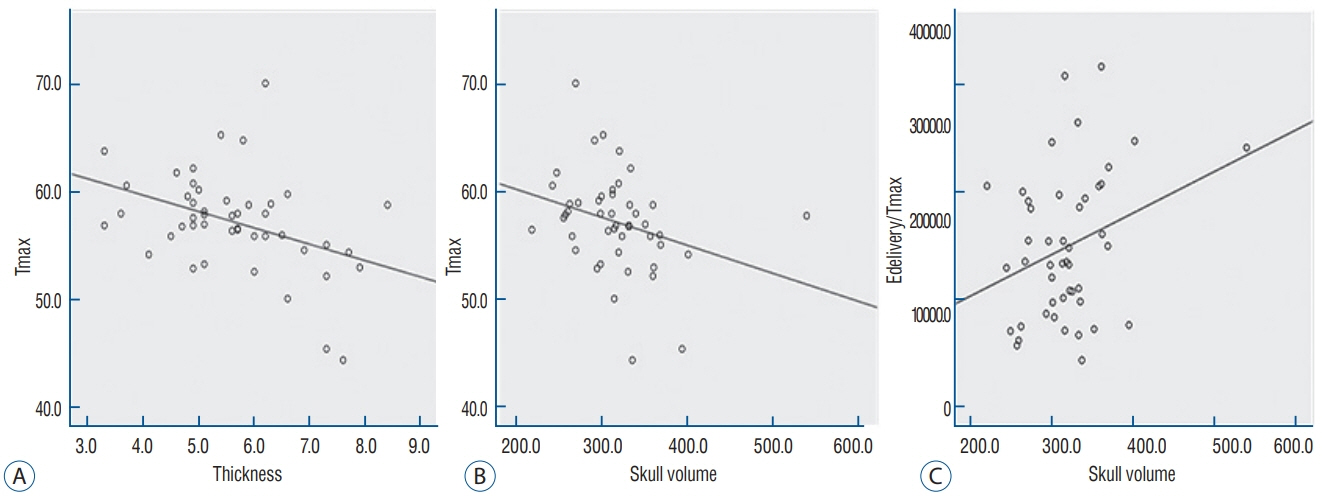
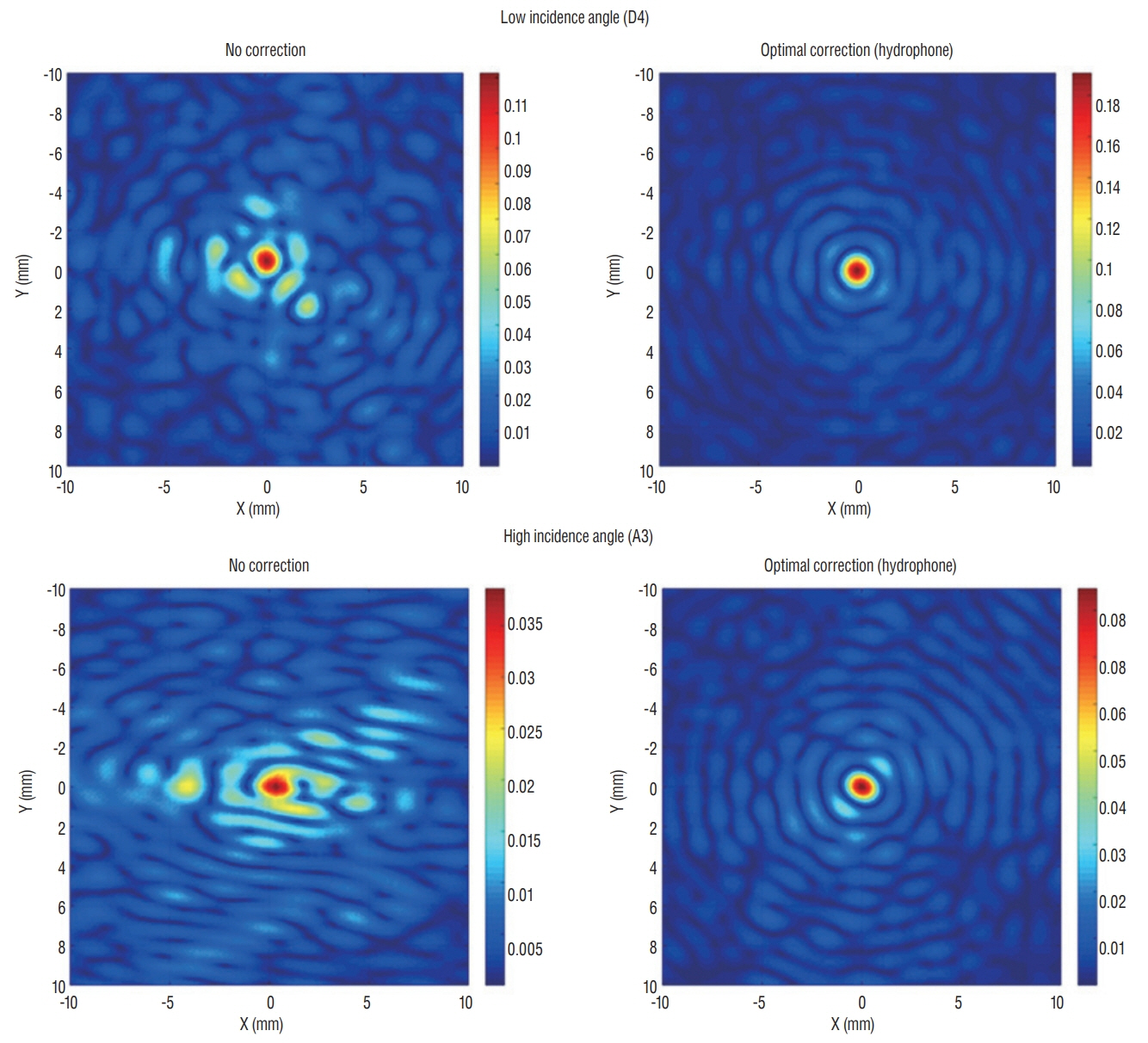
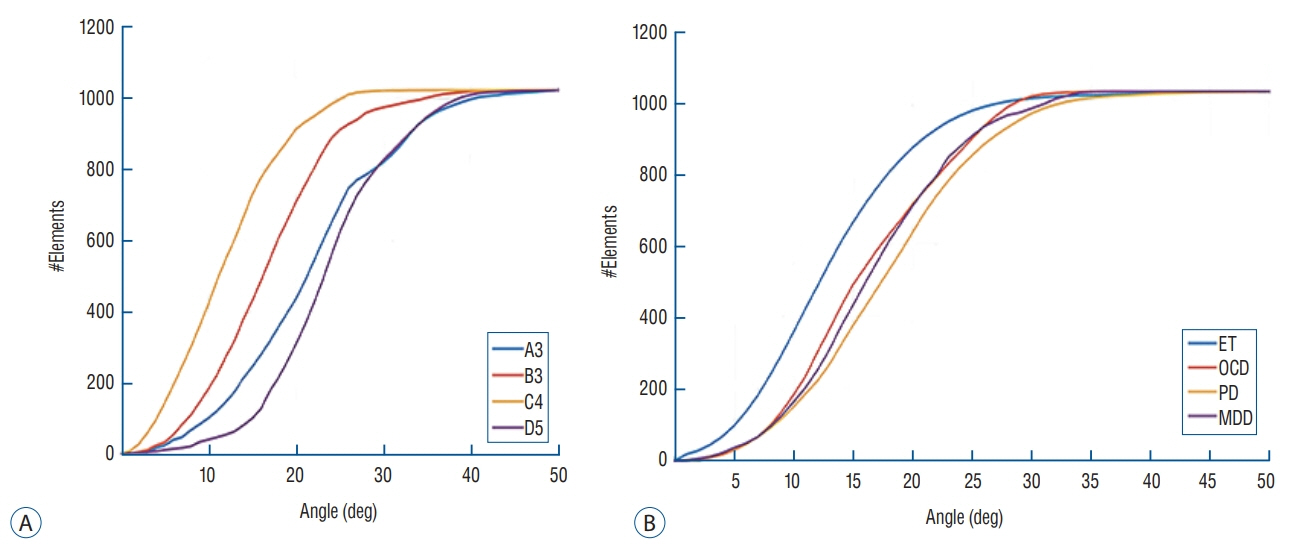
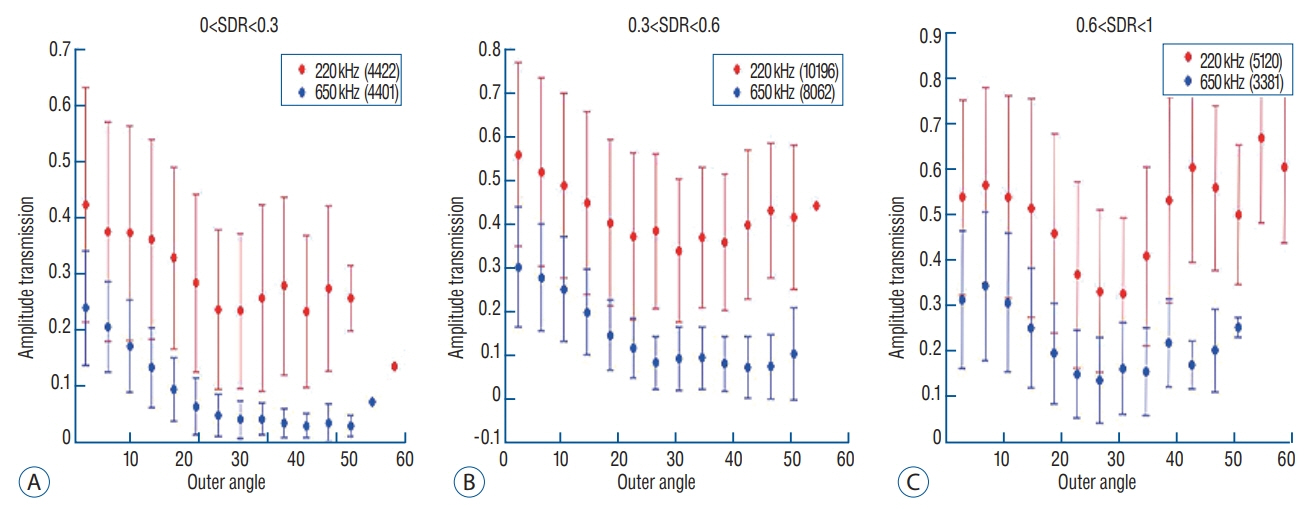
Skull experiments identified three important factors related skull penetration of ultrasound, including skull density ratio (SDR), skull volume, and incidence angle of the acoustic rays against the skull surface. In clinical results, SDR and skull volume correlated with maximal temperature (Tmax) and energy requirement to achieve Tmax (p<0.05). In addition, considering the incidence angle determined by brain target location, less energy was required to reach Tmax in the central, rather than lateral targets particularly when compared between thalamotomy and capsulotomy (p<0.05).
CONCLUSION
This study reconfirmed previously identified skull factors, including SDR and skull volume, for successful MRgFUS; it identified an additional factor, incidence angle of acoustic rays against the skull surface. To guarantee successful transcranial MRgFUS treatment without suffering these various skull issues, further technical improvements are required.
MeSH Terms
Figure
Reference
-
References
1. Bond AE, Shah BB, Huss DS, Dallapiazza RF, Warren A, Harrison MB, et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant parkinson disease: a randomized clinical trial. JAMA Neurol. 74:1412–1418. 2017.
Article2. Chang WS, Jung HH, Kweon EJ, Zadicario E, Rachmilevitch I, Chang JW. Unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor: practices and clinicoradiological outcomes. J Neurol Neurosurg Psychiatry. 86:257–264. 2015.
Article3. Chang WS, Jung HH, Zadicario E, Rachmilevitch I, Tlusty T, Vitek S, et al. Factors associated with successful magnetic resonance-guided focused ultrasound treatment: efficiency of acoustic energy delivery through the skull. J Neurosurg. 124:411–416. 2016.
Article4. Chazen JL, Sarva H, Stieg PE, Min RJ, Ballon DJ, Pryor KO, et al. Clinical improvement associated with targeted interruption of the cerebellothalamic tract following MR-guided focused ultrasound for essential tremor. J Neurosurg. 129:315–323. 2018.
Article5. Chen PY, Hsieh HY, Huang CY, Lin CY, Wei KC, Liu HL. Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J Transl Med. 13:93. 2015.
Article6. Choi JJ, Wang S, Brown TR, Small SA, Duff KE, Konofagou EE. Noninvasive and transient blood-brain barrier opening in the hippocampus of alzheimer’s double transgenic mice using focused ultrasound. Ultrason Imaging. 30:189–200. 2008.
Article7. Chu PC, Liu HL, Lai HY, Lin CY, Tsai HC, Pei YC. Neuromodulation accompanying focused ultrasound-induced blood-brain barrier opening. Sci Rep. 5:15477. 2015.
Article8. Clement GT, White J, Hynynen K. Investigation of a large-area phased array for focused ultrasound surgery through the skull. Phys Med Biol. 45:1071–1083. 2000.
Article9. Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol. 47:1219–1236. 2002.
Article10. Dobrakowski PP, Machowska-Majchrzak AK, Labuz-Roszak B, Majchrzak KG, Kluczewska E, Pierzchala KB. Mr-guided focused ultrasound: a new generation treatment of parkinson’s disease, essential tremor and neuropathic pain. Interv Neuroradiol. 20:275–282. 2014.
Article11. Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 375:730–739. 2016.
Article12. Fry FJ, Ades HW, Fry WJ. Production of reversible changes in the central nervous system by ultrasound. Science. 127:83–84. 1958.
Article13. Fry WJ, Mosberg WH Jr, Barnard JW, Fry FJ. Production of focal destructive lesions in the central nervous system with ultrasound. J Neurosurg. 11:471–478. 1954.
Article14. Fry WJ, Barnard JW, Fry EJ, Krumins RF, Brennan JF. Ultrasonic lesions in the mammalian central nervous system. Science. 122:517–518. 1955.
Article15. Gallay MN, Moser D, Rossi F, Pourtehrani P, Magara AE, Kowalski M, et al. Incisionless transcranial mr-guided focused ultrasound in essential tremor: cerebellothalamic tractotomy. J Ther Ultrasound. 4:5. 2016.
Article16. Hynynen K, Clement GT, McDannold N, Vykhodtseva N, King R, White PJ, et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: a preliminary rabbit study with ex vivo human skulls. Magn Reson Med. 52:100–107. 2004.
Article17. Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 34:814–823. 1995.
Article18. Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus. 32:E1. 2012.
Article19. Jung HH, Kim SJ, Roh D, Chang JG, Chang WS, Kweon EJ, et al. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive-compulsive disorder: a proof-ofconcept study. Mol Psychiatry. 20:1205–1211. 2015.
Article20. Kim M, Kim CH, Jung HH, Kim SJ, Chang JW. Treatment of major depressive disorder via magnetic resonance-guided focused ultrasound surgery. Biol Psychiatry. 83:e17–e18. 2018.
Article21. Kim SJ, Noh DY, Jung HH, Chang WS, Chang JW, Kim H. PT620. A pilot study of bilateral thermal capsulotomy with focused ultrasound for treatment-refractory (MRgFUS) obsessive-compulsive disorder. Int J Neuropsychopharmacol. 19(Suppl 1):27–28. 2016.
Article22. Kuroda K, Oshio K, Chung AH, Hynynen K, Jolesz FA. Temperature mapping using the water proton chemical shift: a chemical shift selective phase mapping method. Magn Reson Med. 38:845–851. 1997.
Article23. Martínez-Fernández R, Rodríguez-Rojas R, Del Álamo M, Hernández-Fernández F, Pineda-Pardo JA, Dileone M, et al. Focused ultrasound subthalamotomy in patients with asymmetric parkinson’s disease: a pilot study. Lancet Neurol. 17:54–63. 2018.
Article24. Mueller JK, Ai L, Bansal P, Legon W. Numerical evaluation of the skull for human neuromodulation with transcranial focused ultrasound. J Neural Eng. 14:066012. 2017.
Article25. Na YC, Chang WS, Jung HH, Kweon EJ, Chang JW. Unilateral magnetic resonance-guided focused ultrasound pallidotomy for parkinson disease. Neurology. 85:549–551. 2015.
Article26. Sun J, Hynynen K. The potential of transskull ultrasound therapy and surgery using the maximum available skull surface area. J Acoust Soc Am. 105:2519–2527. 1999.
Article27. Tsai SJ. Transcranial focused ultrasound as a possible treatment for major depression. Med Hypotheses. 84:381–383. 2015.
Article28. Tung YS, Marquet F, Teichert T, Ferrera V, Konofagou EE. Feasibility of noninvasive cavitation-guided blood-brain barrier opening using focused ultrasound and microbubbles in nonhuman primates. Appl Phys Lett. 98:163704. 2011.29. Wegener N, Kaegi G, Bauer R, Werner B, Martin E, Schreglmann SR, et al. MR-guided high intensity focused ultrasound in Parkinson’s disease: a series of 5 cases. Mov Disord. 31:S2. 2016.30. Wei KC, Chu PC, Wang HY, Huang CY, Chen PY, Tsai HC, et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One. 8:e58995. 2013.
Article31. Weintraub D, Elias WJ. The emerging role of transcranial magnetic resonance imaging-guided focused ultrasound in functional neurosurgery. Mov Disord. 32:20–27. 2017.
Article32. Wu SK, Chu PC, Chai WY, Kang ST, Tsai CH, Fan CH, et al. Characterization of different microbubbles in assisting focused ultrasound-induced blood-brain barrier opening. Sci Rep. 7:46689. 2017.
Article33. Zaaroor M, Sinai A, Goldsher D, Eran A, Nassar M, Schlesinger I. Magnetic resonance-guided focused ultrasound thalamotomy for tremor: a report of 30 parkinson’s disease and essential tremor cases. J Neurosurg. 128:202–210. 2018.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Skull Factors Affecting Outcomes of Magnetic Resonance-Guided Focused Ultrasound for Patients with Essential Tremor
- Magnetic Resonance-Guided Focused Ultrasound in Neurosurgery: Taking Lessons from the Past to Inform the Future
- Focused ultrasound treatment for central nervous system disease: neurosurgeon's perspectives
- Efficacy, Efficiency, and Safety of Magnetic Resonance-Guided High-Intensity Focused Ultrasound for Ablation of Uterine Fibroids: Comparison with Ultrasound-Guided Method
- A review of low-intensity focused ultrasound for neuromodulation