J Korean Neurosurg Soc.
2019 Nov;62(6):649-660. 10.3340/jkns.2019.0132.
Characteristics and Clinical Course of Fusiform Middle Cerebral Artery Aneurysms According to Location, Size, and Configuration
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. nsbang@snubh.org, nsmidget@gmail.com
- 2Department of Neurosurgery, Chungnam National University Hospital, Daejeon, Korea.
- KMID: 2463657
- DOI: http://doi.org/10.3340/jkns.2019.0132
Abstract
OBJECTIVE
To analyze the angiographic features and clinical course, including treatment outcomes and the natural course, of fusiform middle cerebral artery aneurysms (FMCAAs) according to their location, size, and configuration.
METHODS
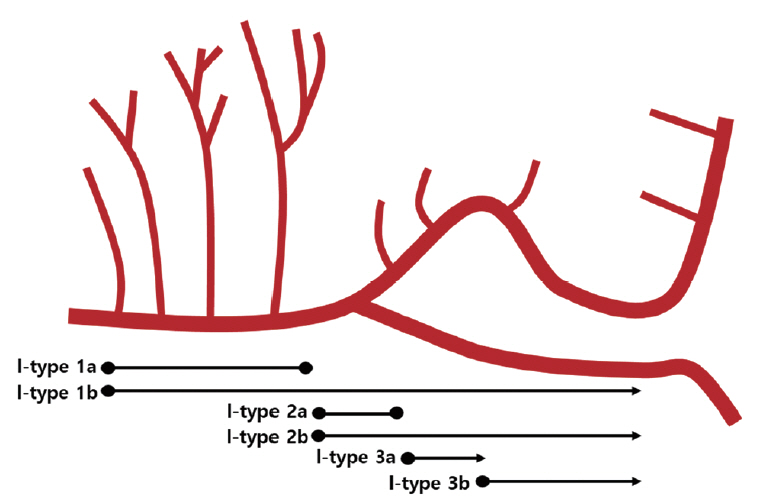
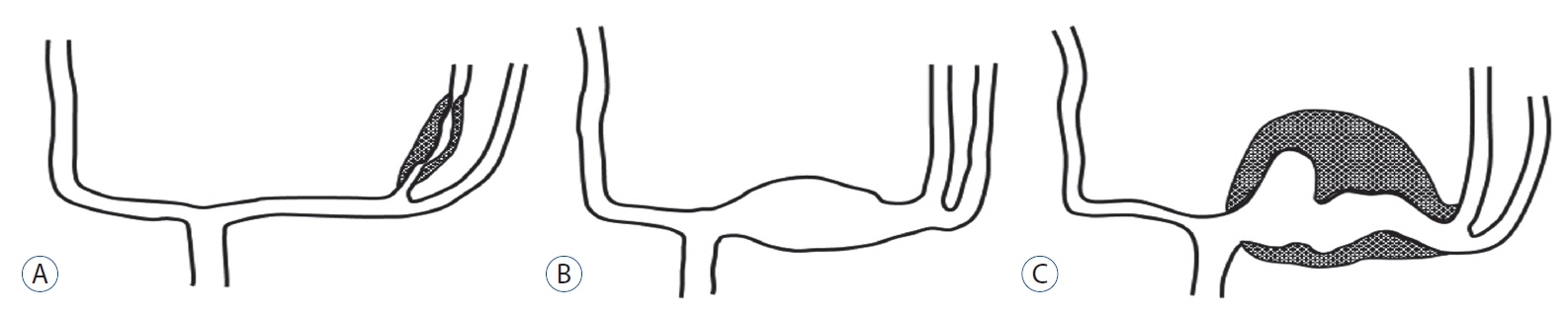
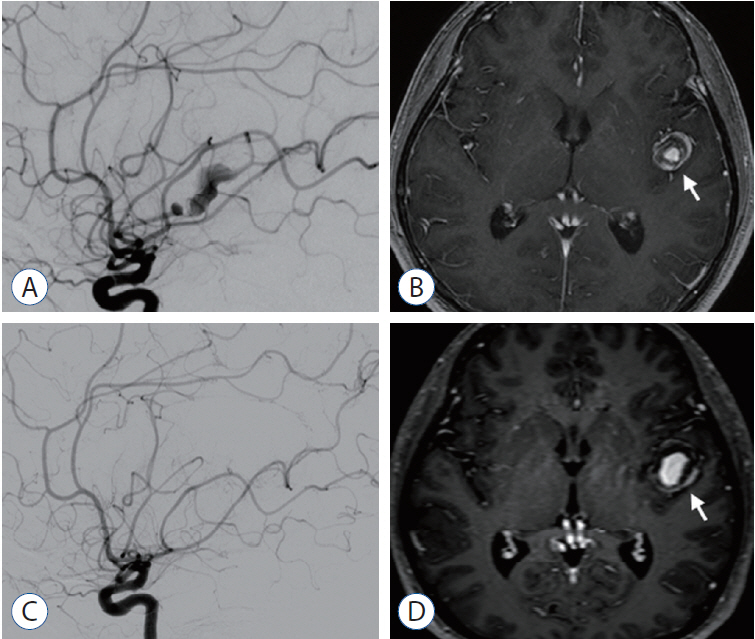
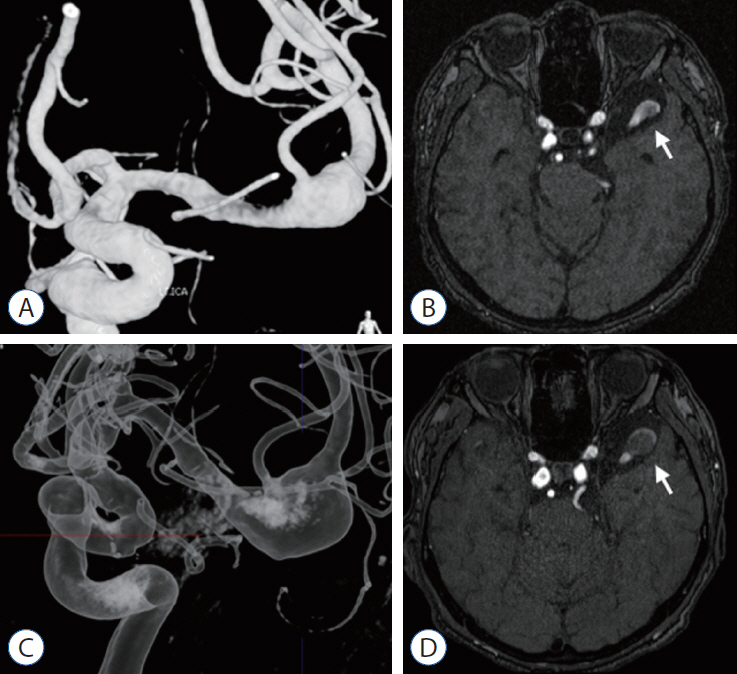
We reviewed the literature on adult cases of FMCAAs published from 1980 to 2018; from 25 papers, 112 FMCAA cases, for which the location, size, and configuration could be identified, were included in this study. Additionally, 33 FMCAA cases in our hospital were included, from which 16 were assigned to the observation group. Thus, a total of 145 adult FMCAA cases were included. We classified the FMCAAs according to their location (l-type 1, beginning from prebifurcation; l-type 2, beginning from bifurcation; l-type 3, beginning from postbifurcation), size (small, <10 mm; large, ≥10 mm; giant, ≥25 mm), and configuration (c-type 1, classic dissecting aneurysm; c-type 2, segmental ectasia; c-type 3, dolichoectatic dissecting aneurysm).
RESULTS
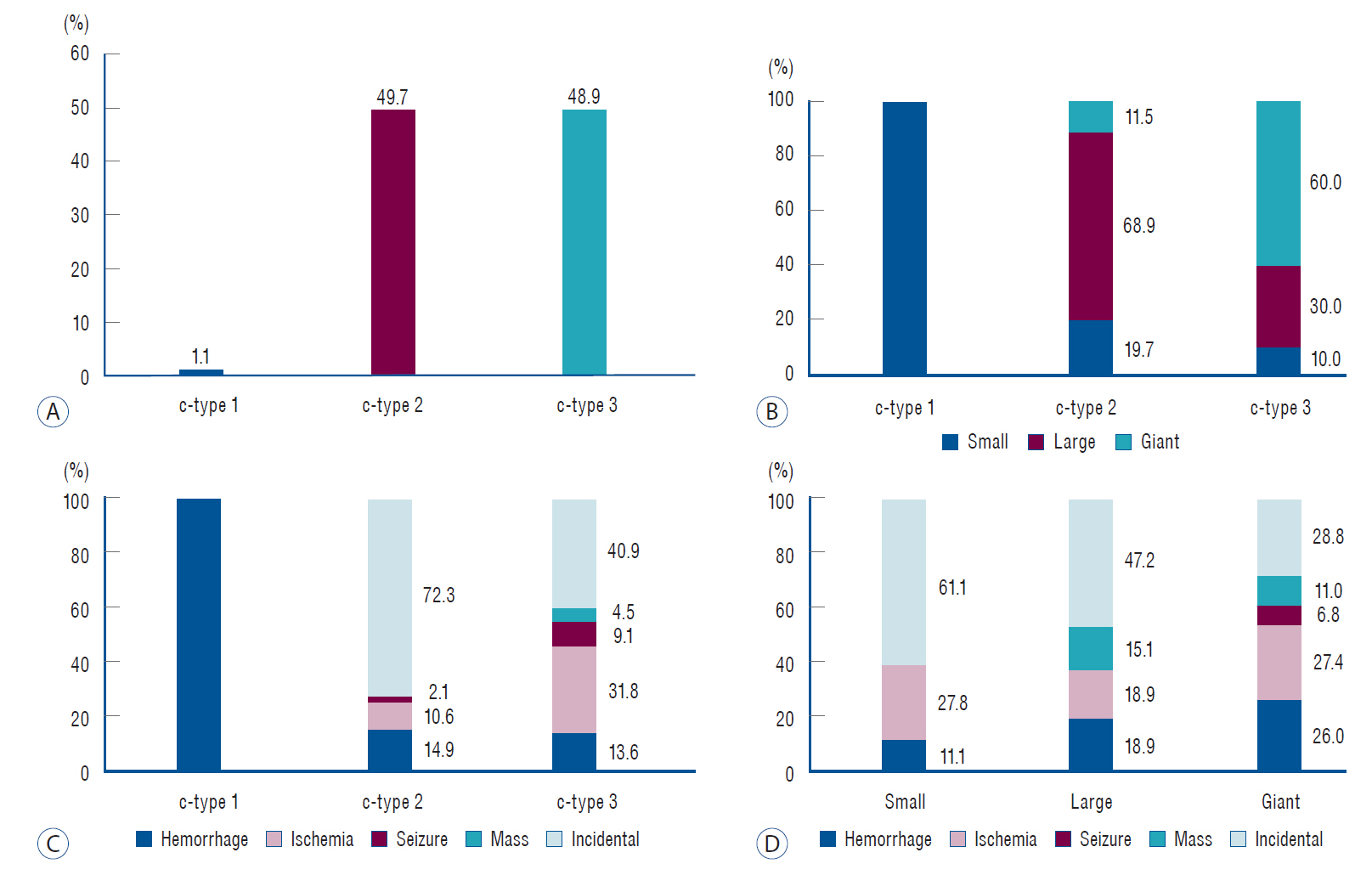
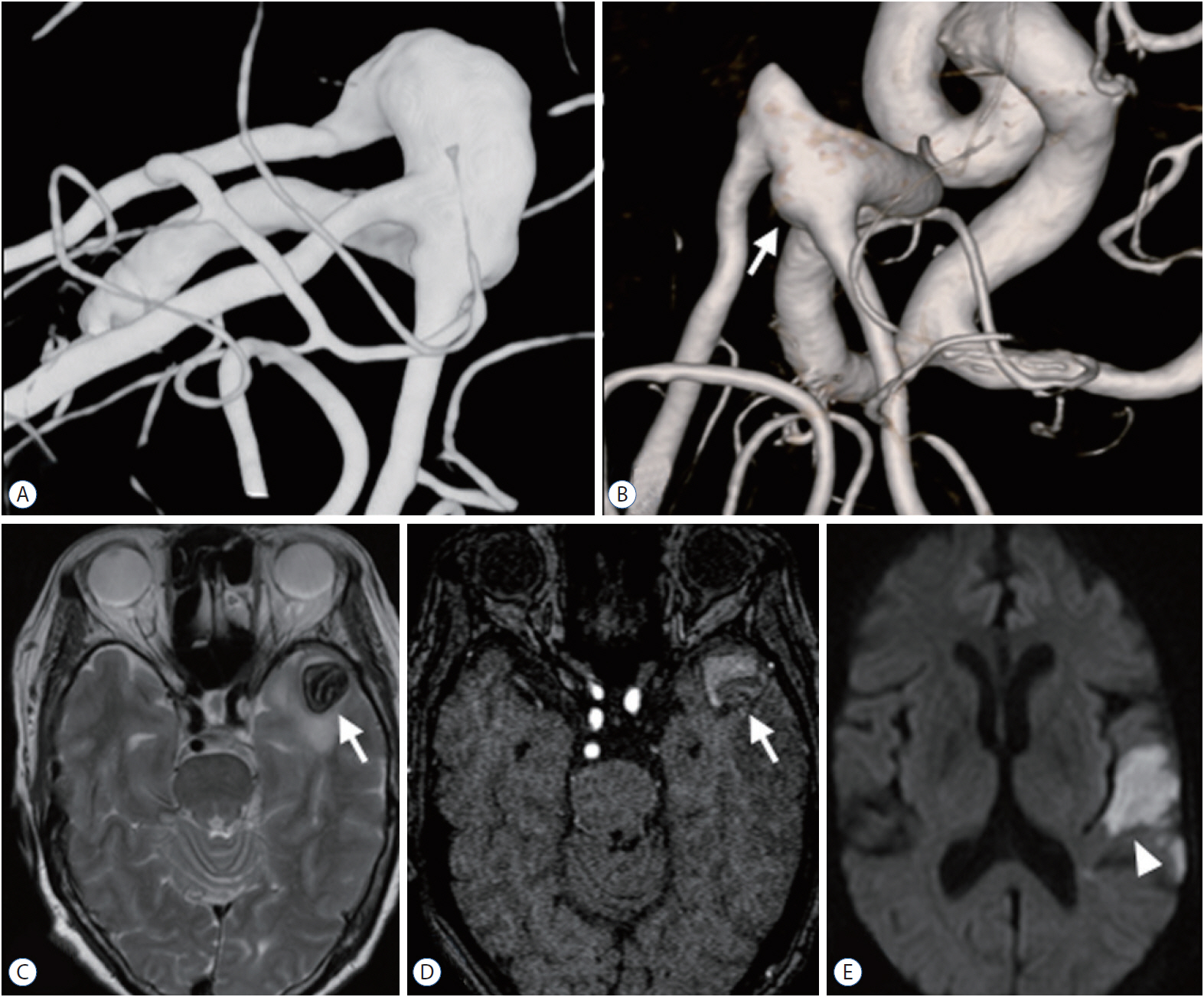
The c-type 3 was more commonly diagnosed with ischemic symptoms (31.8%) than hemorrhage (13.6%), while 40.9% were found accidentally. In contrast, c-type 2 was more commonly diagnosed with hemorrhagic symptoms (14.9%) than ischemic symptoms (10.6%), and 72.3% were accidentally discovered. According to location, ischemic symptoms and hemorrhage were the most frequent symptoms in l-type 1 (28.6%) and l-type 3 (34.6%), respectively. Most of l-type 2 FMCAAs were found incidentally (68.4%). Based on the size of FMCAAs, only 11.1% of small aneurysms were found to be hemorrhagic, while 18.9% and 26.0% of large and giant aneurysms were hemorrhagic, respectively. Although four aneurysms of the 16 FMCAAs in the observation group increased in size and one aneurysm decreased in size during the observation period, no rupture was seen in any case and there were no significant predictors of aneurysm enlargement. Of 104 FMCAAs treated, 14 cases (13.5%) were aggravated than before surgery and all the aggravated cases were l-type 1.
CONCLUSION
While ischemic symptoms occurred more frequently in l-type 1 and c-type 3, hemorrhagic rather than ischemic symptoms occurred more frequently in l-type 3 and c-type 2. In case of l-type 1 FMCAAs, more caution is required in determining the treatment due to the relatively high complication rate.
MeSH Terms
Figure
Cited by 1 articles
-
Microsurgical treatment of distal middle cerebral artery aneurysm: A single-center review
Taehoon Jang, Sung-Tae Kim, Jin Lee, Won-Hee Lee, Keun-Soo Lee, Se-Young Pyo, Junghae Ko, Hangwoo Lee, Yeong Gyun Jeong
J Cerebrovasc Endovasc Neurosurg. 2024;26(1):37-45. doi: 10.7461/jcen.2023.E2023.06.005.
Reference
-
References
1. Alturki AY, Schmalz PGR, Ogilvy CS, Thomas AJ. Sequential coiling-assisted deployment of flow diverter for treatment of fusiform middle cerebral artery aneurysms. Oper Neurosurg (Hagerstown). 15:E13–E18. 2018.
Article2. Al-Yamany M, Ross IB. Giant fusiform aneurysm of the middle cerebral artery: successful Hunterian ligation without distal bypass. Br J Neurosurg. 12:572–575. 1998.
Article3. Anson JA, Lawton MT, Spetzler RF. Characteristics and surgical treatment of dolichoectatic and fusiform aneurysms. J Neurosurg. 84:185–193. 1996.
Article4. Borzone M, Altomonte M, Baldini M, Silvestro C, Rivano C. Giant fusiform aneurysm in middle cerebral artery branches: a report of two cases and a review of the literature. Acta Neurochir (Wien). 125:184–187. 1993.
Article5. Cohen MM, Hemalatha CP, D’Addario RT, Goldman HW. Embolization from a fusiform middle cerebral artery aneurysm. Stroke. 11:158–161. 1980.
Article6. Day AL, Gaposchkin CG, Yu CJ, Rivet DJ, Dacey RG Jr. Spontaneous fusiform middle cerebral artery aneurysms: characteristics and a proposed mechanism of formation. J Neurosurg. 99:228–240. 2003.
Article7. Devulapalli KK, Chowdhry SA, Bambakidis NC, Selman W, Hsu DP. Endovascular treatment of fusiform intracranial aneurysms. J Neurointerv Surg. 5:110–116. 2013.
Article8. Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 87:141–162. 1997.
Article9. Durst CR, Hixson HR, Schmitt P, Gingras JM, Crowley RW. Endovascular treatment of a fusiform aneurysm at the M3-M4 junction of the middle cerebral artery using the pipeline embolization device. World Neurosurg. 86:511.e1–e4. 2016.
Article10. Echiverri HC, Rubino FA, Gupta SR, Gujrati M. Fusiform aneurysm of the vertebrobasilar arterial system. Stroke. 20:1741–1747. 1989.
Article11. Hanel RA, Spetzler RF. Surgical treatment of complex intracranial aneurysms. Neurosurgery. 62:1289–1297. discussion 1297-1299. 2008.
Article12. Horie N, Takahashi N, Furuichi S, Mori K, Onizuka M, Tsutsumi K, et al. Giant fusiform aneurysms in the middle cerebral artery presenting with hemorrhages of different origins. Report of three cases and review of the literature. J Neurosurg. 99:391–396. 2003.
Article13. Horowitz MB, Yonas H, Jungreis C, Hung TK. Management of a giant middle cerebral artery fusiform serpentine aneurysm with distal clip application and retrograde thrombosis: case report and review of the literature. Surg Neurol. 41:221–225. 1994.
Article14. Hrbác T, Drábek P, Klement P, Procházka V. A combined approach to treatment of the dissecting middle cerebral artery fusiform aneurysm. A case report. Interv Neuroradiol. 15:349–354. 2009.
Article15. Jeong SM, Kang SH, Lee NJ, Lim DJ. Stent-assisted coil embolization for the proximal middle cerebral artery fusiform aneurysm. J Korean Neurosurg Soc. 47:406–408. 2010.
Article16. Kalani MY, Zabramski JM, Hu YC, Spetzler RF. Extracranial-intracranial bypass and vessel occlusion for the treatment of unclippable giant middle cerebral artery aneurysms. Neurosurgery. 72:428–435. discussion 435-436. 2013.
Article17. Kivipelto L, Niemelä M, Meling T, Lehecka M, Lehto H, Hernesniemi J. Bypass surgery for complex middle cerebral artery aneurysms: impact of the exact location in the MCA tree. J Neurosurg. 120:398–408. 2014.
Article18. Kobayashi N, Murayama Y, Yuki I, Ishibashi T, Ebara M, Arakawa H, et al. Natural course of dissecting vertebrobasilar artery aneurysms without stroke. AJNR Am J Neuroradiol. 35:1371–1375. 2014.
Article19. Marinkovic S, Gibo H, Milisavljevic M, Cetkovic M. Anatomic and clinical correlations of the lenticulostriate arteries. Clin Anat. 14:190–195. 2001.
Article20. Martin L, Reza D, Jaakko R, Rossana R, Riku K, Mika N, et al. Surgical management of aneurysms of the middle cerebral artery : Schmidek and Sweet Operative Neurosurgical Techniques, ed 6. Beijing: Elsevier;2012. p. 897–913.21. Miller MT, Pasquale M, Kurek S, White J, Martin P, Bannon K, et al. Initial head computed tomographic scan characteristics have a linear relationship with initial intracranial pressure after trauma. J Trauma. 56:967–972. discussion 972-973. 2004.
Article22. Mizutani T, Kojima H. Clinicopathological features of non-atherosclerotic cerebral arterial trunk aneurysms. Neuropathology. 20:91–97. 2000.
Article23. Monteith SJ, Tsimpas A, Dumont AS, Tjoumakaris S, Gonzalez LF, Rosenwasser RH, et al. Endovascular treatment of fusiform cerebral aneurysms with the Pipeline Embolization Device. J Neurosurg. 120:945–954. 2014.
Article24. Mrak G, Duric KS, Nemir J. Middle cerebral artery fusiform aneurysm presented with stroke and delayed subarachnoid hemorrhage trapping, thrombectomy, and bypass. Surg Neurol Int. 7:S209–S213. 2016.
Article25. Nakajima H, Kamiyama H, Nakamura T, Takizawa K, Tokugawa J, Ohata K. Direct surgical treatment of giant middle cerebral artery aneurysms using microvascular reconstruction techniques. Neurol Med Chir (Tokyo). 52:56–61. 2012.
Article26. Nakatomi H, Segawa H, Kurata A, Shiokawa Y, Nagata K, Kamiyama H, et al. Clinicopathological study of intracranial fusiform and dolichoectatic aneurysms : insight on the mechanism of growth. Stroke. 31:896–900. 2000.
Article27. Nasr DM, Flemming KD, Lanzino G, Cloft HJ, Kallmes DF, Murad MH, et al. Natural history of vertebrobasilar dolichoectatic and fusiform aneurysms: a systematic review and meta-analysis. Cerebrovasc Dis. 45:68–77. 2018.
Article28. Niikawa S, Yamada J, Sumi Y, Yamakawa H. Dissecting aneurysm of the middle cerebral artery manifesting as subarachnoid hemorrhage and hemorrhagic infarctions--case report. Neurol Med Chir (Tokyo). 42:62–66. 2002.
Article29. Park KW, Park JS, Hwang SC, Im SB, Shin WH, Kim BT. Vertebral artery dissection: natural history, clinical features and therapeutic considerations. J Korean Neurosurg Soc. 44:109–115. 2008.
Article30. Park SH, Yim MB, Lee CY, Kim E, Son EI. Intracranial fusiform aneurysms: it’s pathogenesis, clinical characteristics and managements. J Korean Neurosurg Soc. 44:116–123. 2008.
Article31. Pumar JM, Lete I, Pardo MI, Vázquez-Herrero F, Blanco M. LEO stent monotherapy for the endovascular reconstruction of fusiform aneurysms of the middle cerebral artery. AJNR Am J Neuroradiol. 29:1775–1776. 2008.
Article32. Selviaridis P, Spiliotopoulos A, Antoniadis C, Kontopoulos V, Foroglou G. Fusiform aneurysm of the posterior cerebral artery: report of two cases. Acta Neurochir (Wien). 144:295–299. discussion 299. 2002.33. Seo BR, Kim TS, Joo SP, Lee JM, Jang JW, Lee JK, et al. Surgical strategies using cerebral revascularization in complex middle cerebral artery aneurysms. Clin Neurol Neurosurg. 111:670–675. 2009.
Article34. Tayebi Meybodi A, Huang W, Benet A, Kola O, Lawton MT. Bypass surgery for complex middle cerebral artery aneurysms: an algorithmic approach to revascularization. J Neurosurg. 127:463–479. 2017.
Article35. Tse KM, Chiu P, Lee HP, Ho P. Investigation of hemodynamics in the development of dissecting aneurysm within patient-specific dissecting aneurismal aortas using computational fluid dynamics (CFD) simulations. J Biomech. 44:827–836. 2011.
Article36. Vega-Basulto SD, Silva-Adán S, Laserda-Gallardo A, Peñones-Montero R, Varela-Hernández A. Giant supratentorial intracranial aneurysms. Analysis of 22 cases. Neurocirugia (Astur). 14:16–24. 2003.37. Weddell JC, Kwack J, Imoukhuede PI, Masud A. Hemodynamic analysis in an idealized artery tree: differences in wall shear stress between Newtonian and non-Newtonian blood models. PLoS One. 10:e0124575. 2015.
Article38. Xu F, Xu B, Huang L, Xiong J, Gu Y, Lawton MT. Surgical treatment of large or giant fusiform middle cerebral artery aneurysms: a case series. World Neurosurg. 115:e252–e262. 2018.
Article39. Yasui T, Komiyama M, Iwai Y, Yamanaka K, Nishikawa M, Morikawa T. Evolution of incidentally-discovered fusiform aneurysms of the vertebrobasilar arterial system: neuroimaging features suggesting progressive aneurysm growth. Neurol Med Chir (Tokyo). 41:523–527. discussion 528. 2001.
Article40. Zhu W, Liu P, Tian Y, Gu Y, Xu B, Chen L, et al. Complex middle cerebral artery aneurysms: a new classification based on the angioarchitecture and surgical strategies. Acta Neurochir (Wien). 155:1481–1491. 2013.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intracranial Fusiform Aneurysms: It's Pathogenesis, Clinical Characteristics and Managements
- Application of Fenestrated Clip in the Intracranial Aneurysms: Report of Four Cases
- Sole Stenting Technique for Treatment of Complex Aneurysms
- Aneurysms of the Proximal Anterior Cerebral Artery
- Multiple Aneurysms on the Same Bifurcation Site of the Middle Cerebral Artery