Clin Exp Otorhinolaryngol.
2019 Nov;12(4):376-384. 10.21053/ceo.2018.01592.
Feasibility of Eye Tracking Assisted Vestibular Rehabilitation Strategy Using Immersive Virtual Reality
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Hallym University College of Medicine, Anyang, Korea. skhong96@hallym.ac.kr
- 2Laboratory of Brain and Cognitive Sciences for Convergence Medicine, Hallym University College of Medicine, Anyang, Korea.
- 3Department of Convergence Software, Hallym University, Chuncheon, Korea.
- 4Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2462733
- DOI: http://doi.org/10.21053/ceo.2018.01592
Abstract
OBJECTIVES
Even though vestibular rehabilitation therapy (VRT) using head-mounted display (HMD) has been highlighted recently as a popular virtual reality platform, we should consider that HMD itself do not provide interactive environment for VRT. This study aimed to test the feasibility of interactive components using eye tracking assisted strategy through neurophysiologic evidence.
METHODS
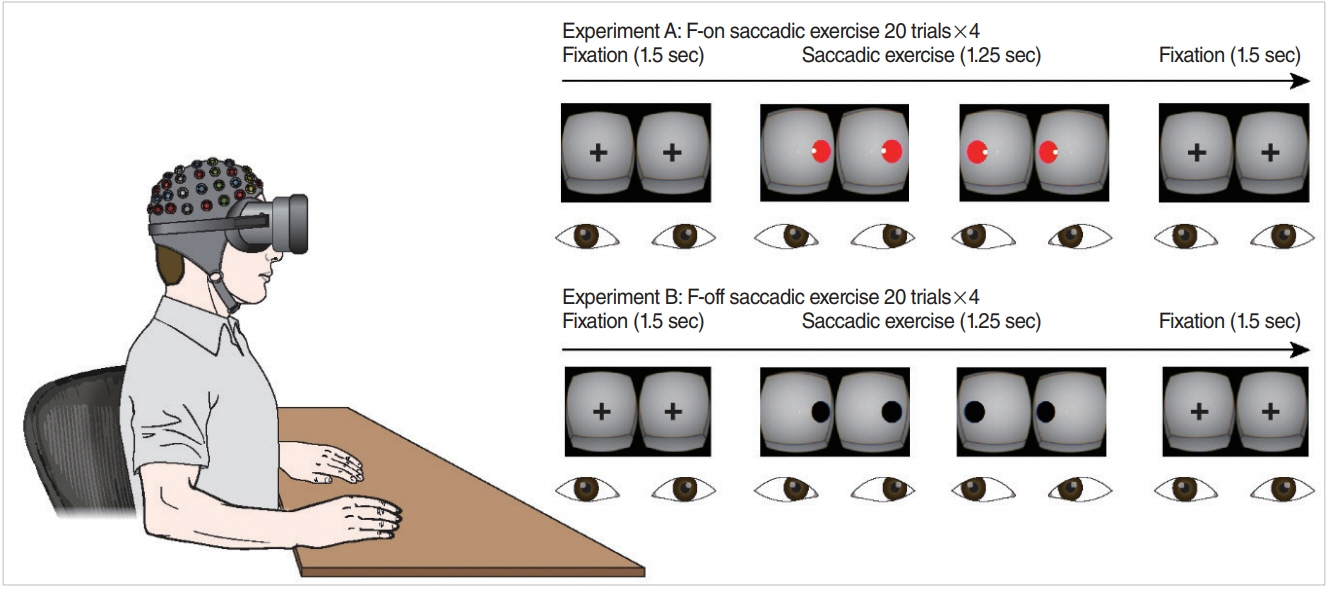
HMD implemented with an infrared-based eye tracker was used to generate a virtual environment for VRT. Eighteen healthy subjects participated in our experiment, wherein they performed a saccadic eye exercise (SEE) under two conditions of feedback-on (F-on, visualization of eye position) and feedback-off (F-off, non-visualization of eye position). Eye position was continuously monitored in real time on those two conditions, but this information was not provided to the participants. Electroencephalogram recordings were used to estimate neural dynamics and attention during SEE, in which only valid trials (correct responses) were included in electroencephalogram analysis.
RESULTS
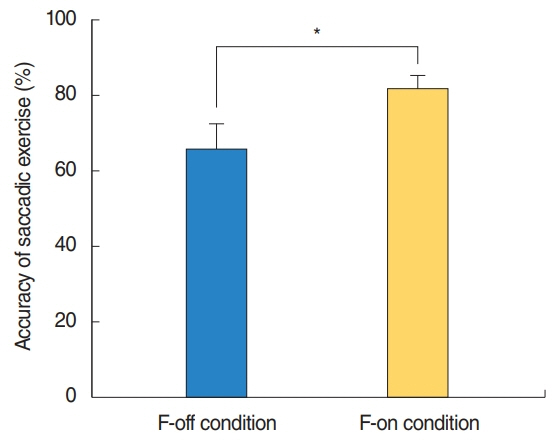
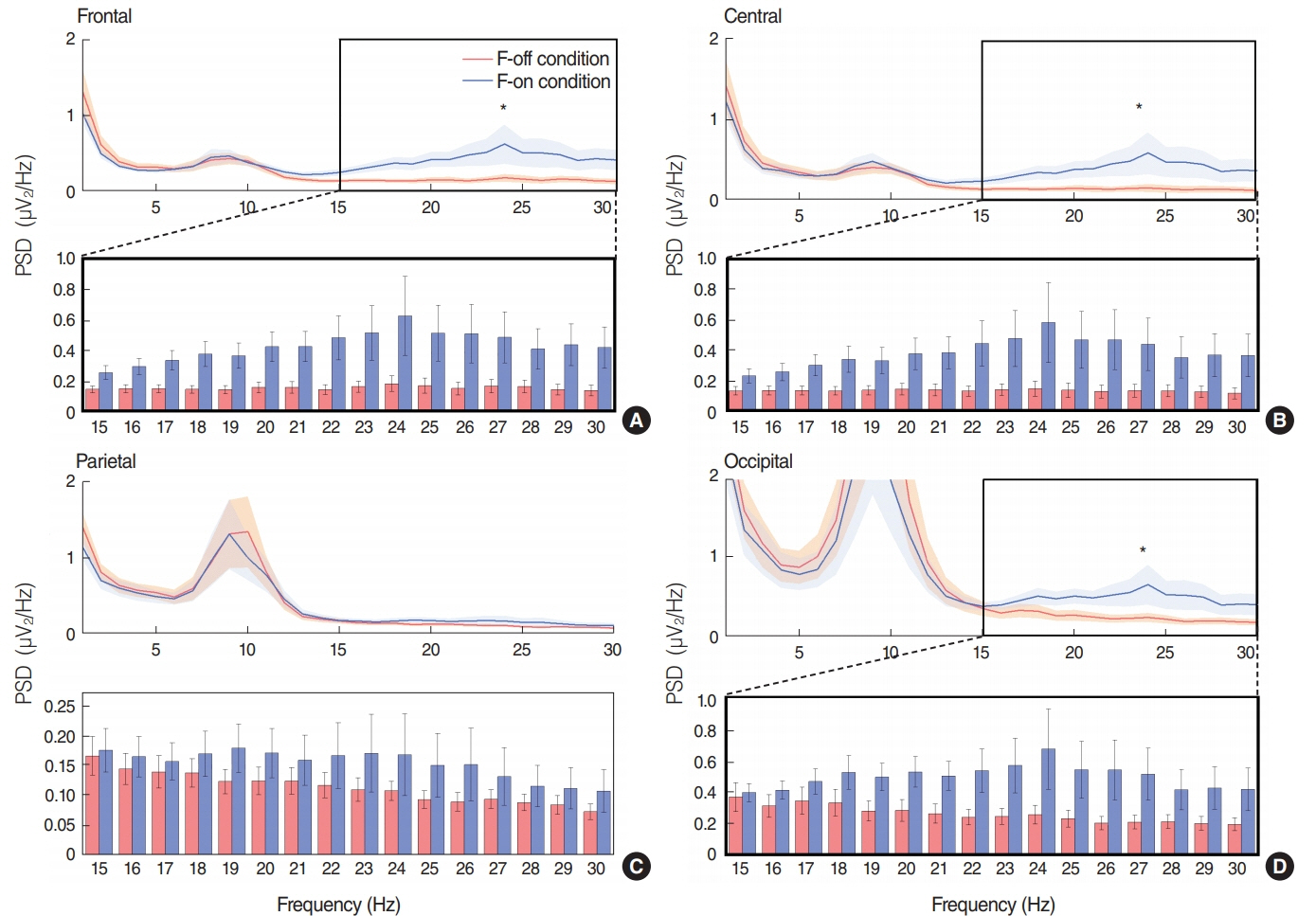
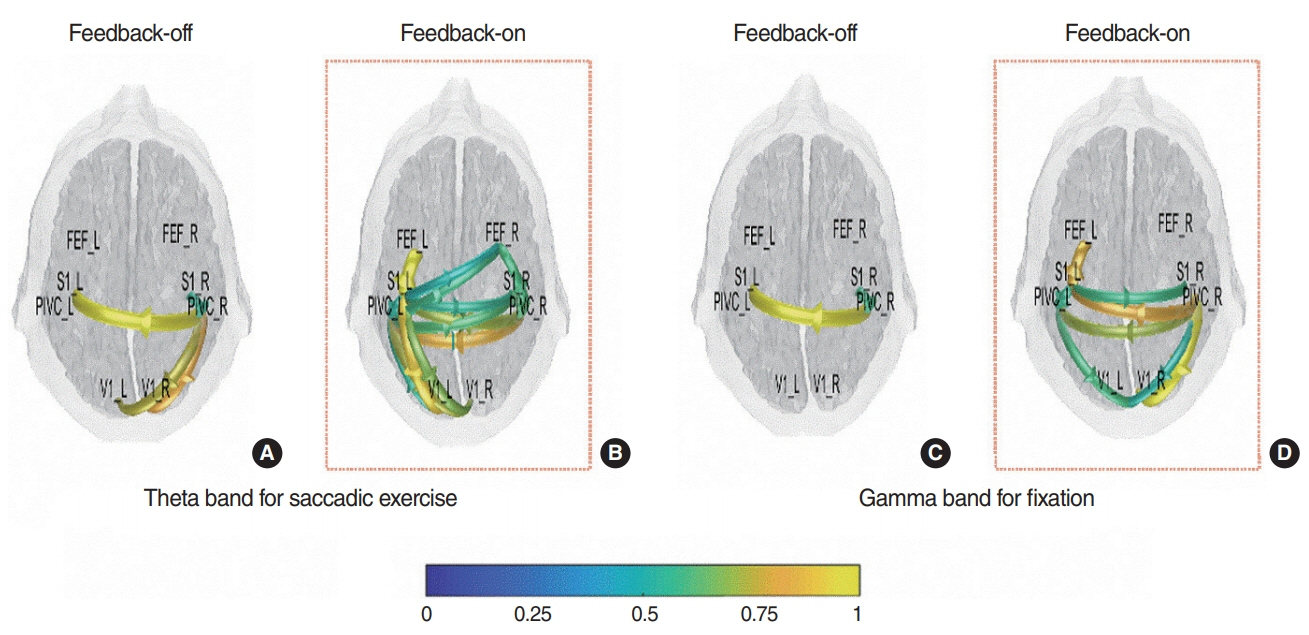
SEE accuracy was higher in the F-on than F-off condition (P=0.039). The power spectral density of beta band was higher in the F-on condition on the frontal (P=0.047), central (P=0.042), and occipital areas (P=0.045). Beta-event-related desynchronization was significantly more pronounced in the F-on (-0.19 on frontal and -0.22 on central clusters) than in the F-off condition (0.23 on frontal and 0.05 on central) on preparatory phase (P=0.005 for frontal and P=0.024 for central). In addition, more abundant functional connectivity was revealed under the F-on condition.
CONCLUSION
Considering substantial gain may come from goal directed attention and activation of brain-network while performing VRT, our preclinical study from SEE suggests that eye tracking algorithms may work efficiently in vestibular rehabilitation using HMD.
Figure
Cited by 2 articles
-
Virtual Reality for Vestibular Rehabilitation
Jae-Jun Song
Clin Exp Otorhinolaryngol. 2019;12(4):329-330. doi: 10.21053/ceo.2019.00983.Recent Advances in the Application of Artificial Intelligence in Otorhinolaryngology-Head and Neck Surgery
Bayu Adhi Tama, Do Hyun Kim, Gyuwon Kim, Soo Whan Kim, Seungchul Lee
Clin Exp Otorhinolaryngol. 2020;13(4):326-339. doi: 10.21053/ceo.2020.00654.
Reference
-
1. Cohen HS, Gottshall KR, Graziano M, Malmstrom EM, Sharpe MH. International survey of vestibular rehabilitation therapists by the Barany Society Ad Hoc Committee on Vestibular Rehabilitation Therapy. J Vestib Res. 2009; 19(1-2):15–20.
Article2. Hecker HC, Haug CO, Herndon JW. Treatment of the vertiginous patient using Cawthorne’s vestibular exercises. Laryngoscope. 1974; Nov. 84(11):2065–72.
Article3. Hillier SL, McDonnell M. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Clin Otolaryngol. 2011; Jun. 36(3):248–9.
Article4. Bamiou DE, Luxon LM. Vertigo: clinical management and rehabilitation. In : Gleeson M, Luxon L, editors. Scott-Brown’s otorhinolaryngology, head and neck surgery. Bora Raton (FL): CRC Press;2008. p. 3791–817.5. Meldrum D, Herdman S, Vance R, Murray D, Malone K, Duffy D, et al. Effectiveness of conventional versus virtual reality-based balance exercises in vestibular rehabilitation for unilateral peripheral vestibular loss: results of a randomized controlled trial. Arch Phys Med Rehabil. 2015; Jul. 96(7):1319–28.
Article6. Pavlou M, Kanegaonkar RG, Swapp D, Bamiou DE, Slater M, Luxon LM. The effect of virtual reality on visual vertigo symptoms in patients with peripheral vestibular dysfunction: a pilot study. J Vestib Res. 2012; 22(5-6):273–81.
Article7. Alahmari KA, Sparto PJ, Marchetti GF, Redfern MS, Furman JM, Whitney SL. Comparison of virtual reality based therapy with customized vestibular physical therapy for the treatment of vestibular disorders. IEEE Trans Neural Syst Rehabil Eng. 2014; Mar. 22(2):389–99.
Article8. Yeh SC, Chen S, Wang PC, Su MC, Chang CH, Tsai PY. Interactive 3-dimensional virtual reality rehabilitation for patients with chronic imbalance and vestibular dysfunction. Technol Health Care. 2014; 22(6):915–21.
Article9. Black FO, Pesznecker SC. Vestibular adaptation and rehabilitation. Curr Opin Otolaryngol Head Neck Surg. 2003; Oct. 11(5):355–60.
Article10. Cohen HS. Disability and rehabilitation in the dizzy patient. Curr Opin Neurol. 2006; Feb. 19(1):49–54.
Article11. Bergeron M, Lortie CL, Guitton MJ. Use of virtual reality tools for vestibular disorders rehabilitation: a comprehensive analysis. Adv Med. 2015; 2015:916735.
Article12. Cooksey FS. Rehabilitation in vestibular injuries. Proc R Soc Lond B Biol Sci. 1946; Mar. 39:273–8.
Article13. Cawthorne T. The physiological basis for head exercises. J Char Soc Physiother. 1944; 3:106–7.14. Badarny S, Aharon-Peretz J, Susel Z, Habib G, Baram Y. Virtual reality feedback cues for improvement of gait in patients with Parkinson’s disease. Tremor Other Hyperkinet Mov (N Y). 2014; Apr. 4:225.15. Hong SK, Park S, Ahn MH, Min BK. Top-down and bottom-up neurodynamic evidence in patients with tinnitus. Hear Res. 2016; Dec. 342:86–100.
Article16. Makeig S, Jung TP, Bell AJ, Ghahremani D, Sejnowski TJ. Blind separation of auditory event-related brain responses into independent components. Proc Natl Acad Sci U S A. 1997; Sep. 94(20):10979–84.
Article17. Bayliss JD, Ballard DH. The effects of eye tracking in a VR helmet on EEG recordings. Rochester (NY): The University of Rochester, Computer Science Department;1998.18. Pelz JB, Hayhoe MM, Ballard DH, Shrivastava A, Bayliss JD, von der Heyde M. Development of a virtual laboratory for the study of complex human behavior. In : Merritt JO, Bolas MT, Fisher SS, editors. Stereoscopic displays and virtual reality systems VI. Bellingham: SPIE;1999.19. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004; Mar. 134(1):9–21.
Article20. Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol. 1993; Apr. 86(4):283–93.
Article21. Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008; May. 58(3):429–41.
Article22. He B, Dai Y, Astolfi L, Babiloni F, Yuan H, Yang L. eConnectome: a MATLAB toolbox for mapping and imaging of brain functional connectivity. J Neurosci Methods. 2011; Feb. 195(2):261–9.
Article23. Sohrabpour A, Ye S, Worrell GA, Zhang W, He B. Noninvasive electromagnetic source imaging and granger causality analysis: an electrophysiological connectome (eConnectome) approach. IEEE Trans Biomed Eng. 2016; Dec. 63(12):2474–87.24. Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994; Mar-Apr. 18(2):192–205.
Article25. Whitney SL, Sparto PJ, Brown KE, Furman JM, Jacobson JL, Redfern MS. The potential use of virtual reality in vestibular rehabilitation: preliminary findings with the BNAVE. J Neurol Phys Ther. 2002; 26(2):72–8.26. Gola M, Magnuski M, Szumska I, Wrobel A. EEG beta band activity is related to attention and attentional deficits in the visual performance of elderly subjects. Int J Psychophysiol. 2013; Sep. 89(3):334–41.
Article27. Kilavik BE, Zaepffel M, Brovelli A, MacKay WA, Riehle A. The ups and downs of β oscillations in sensorimotor cortex. Exp Neurol. 2013; Jul. 245:15–26.28. Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000; Feb. 97(4):1867–72.
Article29. Brinkman L, Stolk A, Dijkerman HC, de Lange FP, Toni I. Distinct roles for alpha- and beta-band oscillations during mental simulation of goal-directed actions. J Neurosci. 2014; Oct. 34(44):14783–92.
Article30. de Lange FP, Jensen O, Bauer M, Toni I. Interactions between posterior gamma and frontal alpha/beta oscillations during imagined actions. Front Hum Neurosci. 2008; Aug. 2:7.
Article31. McFarland DJ, Miner LA, Vaughan TM, Wolpaw JR. Mu and beta rhythm topographies during motor imagery and actual movements. Brain Topogr. 2000; 12(3):177–86.32. Ng TH, Sowman PF, Brock J, Johnson BW. Premovement brain activity in a bimanual load-lifting task. Exp Brain Res. 2011; Jan. 208(2):189–201.
Article33. Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. Beta-band activity during motor planning reflects response uncertainty. J Neurosci. 2010; Aug. 30(34):11270–7.
Article34. Helmchen C, Ye Z, Sprenger A, Munte TF. Changes in resting-state fMRI in vestibular neuritis. Brain Struct Funct. 2014; Nov. 219(6):1889–900.
Article35. Hong SK, Kim JH, Kim HJ, Lee HJ. Changes in the gray matter volume during compensation after vestibular neuritis: a longitudinal VBM study. Restor Neurol Neurosci. 2014; 32(5):663–73.
Article36. Dieterich M, Bauermann T, Best C, Stoeter P, Schlindwein P. Evidence for cortical visual substitution of chronic bilateral vestibular failure (an fMRI study). Brain. 2007; Aug. 130(Pt 8):2108–16.
Article37. Brandt T, Strupp M, Dieterich M. Five keys for diagnosing most vertigo, dizziness, and imbalance syndromes: an expert opinion. J Neurol. 2014; Jan. 261(1):229–31.
Article38. Schubert MC, Zee DS. Saccade and vestibular ocular motor adaptation. Restor Neurol Neurosci. 2010; 28(1):9–18.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical application of virtual reality for vestibular rehabilitation
- Correction: A Fully Immersive Virtual Reality Method for Upper Limb Rehabilitation in Spinal Cord Injury
- Motor Learning by Novel Therapeutic Approaches: Virtual Reality and Robotics
- Virtual Reality Technology Trends in Aeromedical Field
- Satisfaction and Effect Research on Virtual Reality-Based Vestibular Exercise for the Elderly Patients with Chronic Unilateral Vestibulopathy