Clin Orthop Surg.
2019 Sep;11(3):352-360. 10.4055/cios.2019.11.3.352.
Evaluation of Local Recurrence in Giant-Cell Tumor of Bone Treated by Neoadjuvant Denosumab
- Affiliations
-
- 1Department of Musculoskeletal Oncology, HCG Hospital, Bangalore, India. suraj.ortho@gmail.com
- KMID: 2462566
- DOI: http://doi.org/10.4055/cios.2019.11.3.352
Abstract
- BACKGROUND
Giant-cell tumor of bone (GCTB) is a locally aggressive primary benign tumor presenting as an expansile osteolytic lesion affecting the epiphysis of long bones. Denosumab halts the osteolysis by giant cells thereby downstaging the tumor, helping in performing less morbid procedures to remove the tumor. Our aim was to report the incidence of local recurrence (LR) in patients operated following neoadjuvant denosumab, to investigate factors associated with LR following extended curettage for GCTB, and to compare the postoperative functional and oncological outcome of patients operated with and without neoadjuvant denosumab.
METHODS
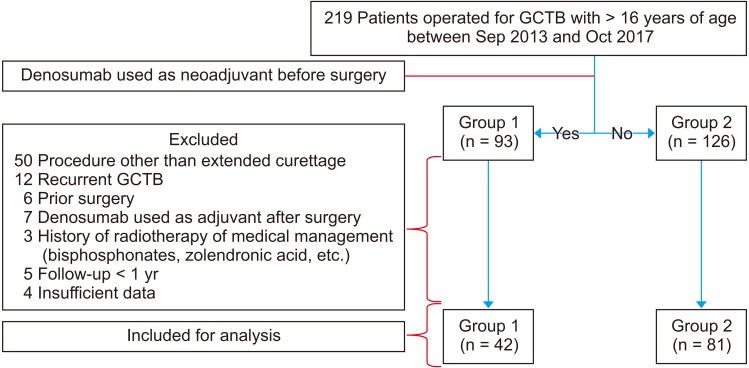
A total of 123 patients with a mean age of 29.6 years undergoing extended curettage for GCTB were retrospectively divided into group 1 receiving neoadjuvant denosumab and group 2 operated without denosumab. The mean follow-up period was 35 months. The perioperative characteristics and outcome were compared between the two groups and the factors for LR of GCTB were analyzed.
RESULTS
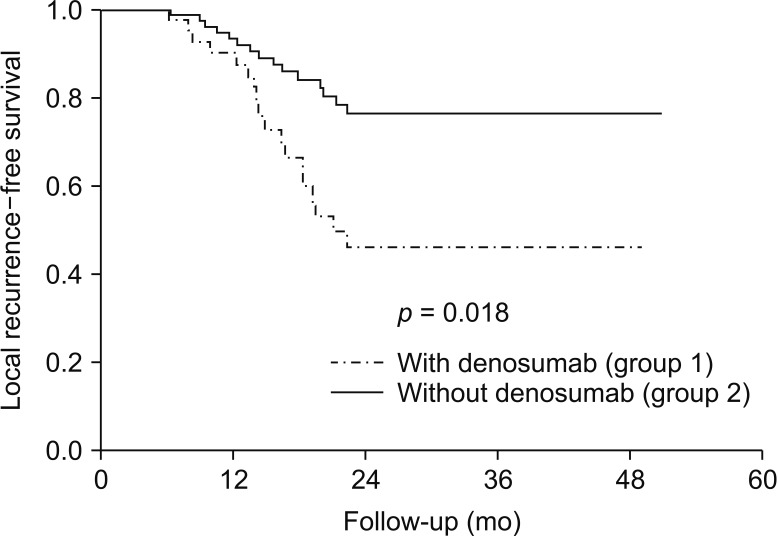
The incidence of LR among patients operated after neoadjuvant denosumab therapy was 42.8% and was significantly high compared to that in patients without denosumab (p < 0.001). On multivariate logistic regression analysis, use of denosumab as a neoadjuvant was the only factor independently associated with LR following surgery (p = 0.002). Patients treated with denosumab had a lower LR-free survival rate (log-rank, p = 0.018).
CONCLUSIONS
Denosumab was independently associated with increased LR following surgery for GCTB. Denosumab has to be used cautiously in patients in whom the burden of downstaging the disease outweighs the possible chance of LR.
Keyword
MeSH Terms
Figure
Reference
-
1. Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol. 2006; 29(1):96–99. PMID: 16462511.
Article2. Rigollino AV, Fernando TS, Tanaka MH, Souza MM. Giant cell tumor locally advanced around the knee: treatment and literature review. Rev Bras Ortop. 2017; 52(4):473–478. PMID: 28884107.
Article3. Lin F, Hu Y, Zhao L, et al. The epidemiological and clinical features of primary giant cell tumor around the knee: a report from the multicenter retrospective study in china. J Bone Oncol. 2016; 5(1):38–42. PMID: 26998425.
Article4. van der Heijden L, Dijkstra PD, Blay JY, Gelderblom H. Giant cell tumour of bone in the denosumab era. Eur J Cancer. 2017; 77:75–83. PMID: 28365529.
Article5. Chakarun CJ, Forrester DM, Gottsegen CJ, Patel DB, White EA, Matcuk GR Jr. Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics. 2013; 33(1):197–211. PMID: 23322837.
Article6. Cowan RW, Singh G. Giant cell tumor of bone: a basic science perspective. Bone. 2013; 52(1):238–246. PMID: 23063845.
Article7. Xu SF, Adams B, Yu XC, Xu M. Denosumab and giant cell tumour of bone-a review and future management considerations. Curr Oncol. 2013; 20(5):e442–e447. PMID: 24155640.
Article8. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013; 14(9):901–908. PMID: 23867211.
Article9. Goldenberg MM. Pharmaceutical approval update. P T. 2013; 38(9):518–524. PMID: 24273397.10. Ueda T, Morioka H, Nishida Y, et al. Objective tumor response to denosumab in patients with giant cell tumor of bone: a multicenter phase II trial. Ann Oncol. 2015; 26(10):2149–2154. PMID: 26205395.
Article11. Hakozaki M, Tajino T, Yamada H, et al. Radiological and pathological characteristics of giant cell tumor of bone treated with denosumab. Diagn Pathol. 2014; 9:111. PMID: 24906559.
Article12. Gaston CL, Grimer RJ, Parry M, et al. Current status and unanswered questions on the use of denosumab in giant cell tumor of bone. Clin Sarcoma Res. 2016; 6(1):15. PMID: 27651889.
Article13. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987; 69(1):106–114. PMID: 3805057.
Article14. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993; (286):241–246. PMID: 8425352.
Article15. Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007; 25(13):1753–1759. PMID: 17470865.
Article16. Balke M, Schremper L, Gebert C, et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008; 134(9):969–978. PMID: 18322700.
Article17. Hu P, Zhao L, Zhang H, et al. Recurrence rates and risk factors for primary giant cell tumors around the knee: a multicentre retrospective study in China. Sci Rep. 2016; 6:36332. PMID: 27827384.
Article18. Gaston CL, Bhumbra R, Watanuki M, et al. Does the addition of cement improve the rate of local recurrence after curettage of giant cell tumours in bone? J Bone Joint Surg Br. 2011; 93(12):1665–1669. PMID: 22161931.
Article19. Kivioja AH, Blomqvist C, Hietaniemi K, et al. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008; 79(1):86–93. PMID: 18283578.
Article20. Prosser GH, Baloch KG, Tillman RM, Carter SR, Grimer RJ. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop Relat Res. 2005; (435):211–218. PMID: 15930941.
Article21. Leggon RE, Zlotecki R, Reith J, Scarborough MT. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res. 2004; (423):196–207. PMID: 15232449.22. Traub F, Singh J, Dickson BC, et al. Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone. Eur J Cancer. 2016; 59:1–12. PMID: 26990281.
Article23. Errani C, Tsukamoto S, Leone G, et al. Denosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettage. J Bone Joint Surg Am. 2018; 100(6):496–504. PMID: 29557866.
Article24. Biscaglia R, Bacchini P, Bertoni F. Giant cell tumor of the bones of the hand and foot. Cancer. 2000; 88(9):2022–2032. PMID: 10813712.
Article25. Rajani R, Schaefer L, Scarborough MT, Gibbs CP. Giant cell tumors of the foot and ankle bones: high recurrence rates after surgical treatment. J Foot Ankle Surg. 2015; 54(6):1141–1145. PMID: 25441851.
Article26. Shigematsu K, Kobata Y, Yajima H, Kawamura K, Maegawa N, Takakura Y. Giant-cell tumors of the carpus. J Hand Surg Am. 2006; 31(7):1214–1219. PMID: 16945730.
Article27. Co HL, Wang EH. Giant cell tumor of the small bones of the foot. J Orthop Surg (Hong Kong). 2018; 26(3):2309499018801168. PMID: 30270796.
Article28. Cheng DD, Hu T, Zhang HZ, Huang J, Yang QC. Factors affecting the recurrence of giant cell tumor of bone after surgery: a clinicopathological study of 80 cases from a single center. Cell Physiol Biochem. 2015; 36(5):1961–1970. PMID: 26202356.
Article29. Mak IW, Evaniew N, Popovic S, Tozer R, Ghert M. A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am. 2014; 96(15):e127. PMID: 25100780.
Article30. Muller DA, Beltrami G, Scoccianti G, Campanacci DA, Franchi A, Capanna R. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol. 2016; 14(1):281. PMID: 27809843.
Article31. Goldschlager T, Dea N, Boyd M, et al. Giant cell tumors of the spine: has denosumab changed the treatment paradigm. J Neurosurg Spine. 2015; 22(5):526–533. PMID: 25700239.
Article32. Deveci MA, Paydas S, Gonlusen G, Ozkan C, Bicer OS, Tekin M. Clinical and pathological results of denosumab treatment for giant cell tumors of bone: prospective study of 14 cases. Acta Orthop Traumatol Turc. 2017; 51(1):1–6. PMID: 27784623.
Article33. Martin-Broto J, Cleeland CS, Glare PA, et al. Effects of denosumab on pain and analgesic use in giant cell tumor of bone: interim results from a phase II study. Acta Oncol. 2014; 53(9):1173–1179. PMID: 24834795.
Article34. Sorensen AL, Hansen RL, Jorgensen PH. Denosumab may be a supplement to the surgical treatment of giant cell tumours of bone. Ugeskr Laeger. 2016; 178(36):pii: V03160204.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Issues on Denosumab Use in Giant Cell Tumor of Bone
- Giant Cell Tumor of the Radius Treated by Massive Resection and fibula Bone graft: One case Report
- Denosumab for Treatment of a Recurrent Cervical Giant-Cell Tumor
- Giant Cell Tumor of Bone: It's fate after bone fraft
- Current Strategy of Chemotherapy for Bone Tumors