Clin Orthop Surg.
2019 Dec;11(4):403-408. 10.4055/cios.2019.11.4.403.
Follow-up of Metal-on-Metal Hip Replacements at a Large District Hospital and the Implementation of Medicines and Healthcare Products Regulatory Agency Guidelines: A Review of 297 Patients
- Affiliations
-
- 1Department of Trauma and Orthopaedics, Blackburn Royal Infirmary, Blackburn, UK. drlukehughes@hotmail.co.uk
- KMID: 2462533
- DOI: http://doi.org/10.4055/cios.2019.11.4.403
Abstract
- BACKGROUND
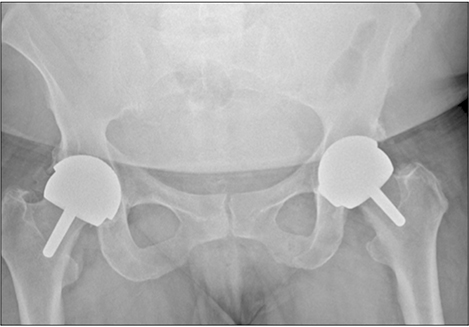
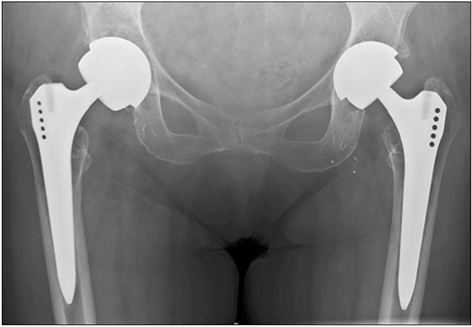
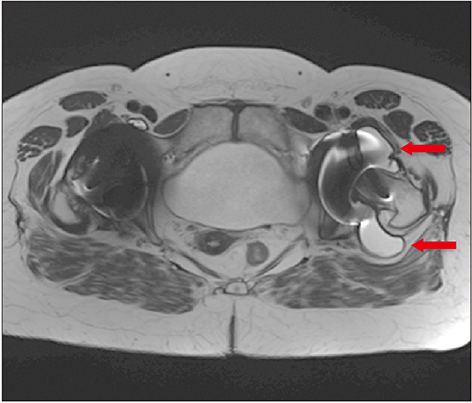
Medicines and Healthcare products Regulatory Agency (MHRA) guidance for patients with metal-on-metal (MoM) hip replacements was provided in 2012 and updated in 2017 to assist in the early detection of soft-tissue reactions due to metal wear debris. A large number of MoM hip replacements were undertaken at our hospital trust. A program of recall for all patients with MoM hip replacements was undertaken and MHRA guidelines were implemented. In this study, we aimed to investigate the effectiveness of the revised MHRA guidelines in the detection of early adverse reactions to metal debris and to re-evaluate the indications for metal artifact reduction sequence magnetic resonance imaging (MARS-MRI) and revision surgery.
METHODS
Identification and recall of all patients with MoM hip replacements from 2001 were conducted by using theatre logs, patient records, clinical coding information, and consultant logbooks. Two senior arthroplasty consultants reviewed X-rays and patient records. Postal questionnaires were forwarded to patients, together with requests for general practitioners to complete cobalt and chromium blood tests. The two consultant-led review of MOM replacements was undertaken with further radiological investigations (X-rays, MARS-MRI) performed according to the 2017 guidance with support of consultant radiologists.
RESULTS
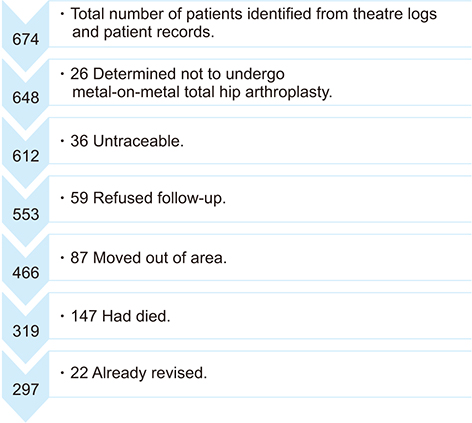
Of 674 identified patients, 297 were available for review: 26 patients did not have MoM implants, 36 were untraceable, 59 refused follow-up, 87 moved out of area, 147 had died, and 22 already had revision. Of 297 patients, 126 were women and 171 were men; age range was 39 to 95 years (mean age, 69 years); 126 had resurfacing and 171 had MoM replacements. Twenty-six patients had elevated metal ions. Thirty-three patients underwent MARS-MRI: MARS-MRI results were positive in 17 and negative in 16. Of 17 patients with positive MARS-MRI, 10 patients were asymptomatic and seven were waiting revision.
CONCLUSIONS
Positive MARS-MRI can often occur in the absence of elevated metal ion levels; elevated blood metal ion levels do not mean MARS-MRI will be positive. All patients with MoM replacements were at risk. It is imperative to assess patients regularly for symptoms that may raise clinical suspicion and maintain a low threshold to performing MARS-MRI.
Keyword
MeSH Terms
Figure
Reference
-
1. HQIP. National joint registry for England, Wales, Northern Ireland and the Isle of Man: 15th annual report 2018. Hemel Hempstead: NJR Service Centre;2018.2. Watters TS, Cardona DM, Menon KS, Vinson EN, Bolognesi MP, Dodd LG. Aseptic lymphocyte-dominated vasculitis-associated lesion: a clinicopathologic review of an underrecognized cause of prosthetic failure. Am J Clin Pathol. 2010; 134(6):886–893.
Article3. Medicines and Healthcare products Regulatory Agency. All metal-on-metal (MoM) hip replacements, health. London: Medicines and Healthcare products Regulatory Agency;2012.4. Berber R, Skinner JA, Hart AJ. Management of metal-on-metal hip implant patients: who, when and how to revise? World J Orthop. 2016; 7(5):272–279.
Article5. Medicines and Healthcare products Regulatory Agency. All metal-on-metal (MoM) hip replacements: updated advice for follow-up of patients. London: Medicines and Healthcare products Regulatory Agency;2017.6. Pickford M. ODEP rating: supporting innovation? Orthopaedic Data Evaluation Panel rating - supporting innovation [Internet]. Nottingham: Northgate Public Services (UK) Ltd.;2013. cited 2019 Sep 1. Available from: http://www.odep.org.uk/ODEPExplained/toPatients.aspx.7. Grammatopoulos G, Pandit H, Kwon YM, et al. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg Br. 2009; 91(8):1019–1024.8. Liddle AD, Satchithananda K, Henckel J, et al. Revision of metal-on-metal hip arthroplasty in a tertiary center: a prospective study of 39 hips with between 1 and 4 years of follow-up. Acta Orthop. 2013; 84(3):237–245.9. Matharu GS, Ostlere SJ, Pandit HG, Murray DW. What is the natural history of asymptomatic pseudotumours in metal-on-metal hip resurfacing patients? Hip Int. 2016; 26(6):522–530.
Article10. Hailer NP, Bengtsson M, Lundberg C, Milbrink J. High metal ion levels after use of the ASR™ device correlate with development of pseudotumors and T cell activation. Clin Orthop Relat Res. 2014; 472(3):953–961.
Article11. Gunther KP, Lutzner J, Hannemann F, et al. Update on metal-on-metal hip joints. Orthopade. 2013; 42(5):373–387.12. Langton DJ, Joyce TJ, Mangat N, et al. Reducing metal ion release following hip resurfacing arthroplasty. Orthop Clin North Am. 2011; 42(2):169–180.
Article13. MacDonald SJ, Brodner W, Jacobs JJ. A consensus paper on metal ions in metal-on-metal hip arthroplasties. J Arthroplasty. 2004; 19(8 Suppl 3):12–16.
Article14. Hart AJ, Sabah SA, Bandi AS, et al. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg Br. 2011; 93(10):1308–1313.
Article15. Sidaginamale RP, Joyce TJ, Lord JK, et al. Blood metal ion testing is an effectivescreening tool to identify poorly performing metal-on-metal bearingsurfaces. Bone Joint Res. 2013; 2(5):84–95.
Article16. Thomas MS, Wimhurst JA, Nolan JF, Toms AP. Imaging metal-on-metal hip replacements: the Norwich experience. HSS J. 2013; 9(3):247–256.
Article17. Siddiqui IA, Sabah SA, Satchithananda K, et al. A comparison of the diagnostic accuracy of MARS MRI and ultrasound of the painful metal-on-metal hip arthroplasty. Acta Orthop. 2014; 85(4):375–382.
Article18. Smith J, Lee D, Bali K, et al. Does bearing size influence metal ion levels in large-head metal-on-metal total hip arthroplasty? A comparison of three total hip systems. J Orthop Surg Res. 2014; 9:3.
Article19. De Smet KA, Van Der Straeten C, Van Orsouw M, Doubi R, Backers K, Grammatopoulos G. Revisions of metal-on-metal hip resurfacing: lessons learned and improved outcome. Orthop Clin North Am. 2011; 42(2):259–269.
Article20. Haddad FS, Thakrar RR, Hart AJ, et al. Metal-on-metal bearings: the evidence so far. J Bone Joint Surg Br. 2011; 93(5):572–579.21. Matharu GS, Pynsent PB, Dunlop DJ. Revision of metal-on-metal hip replacements and resurfacings for adverse reaction to metal debris: a systematic review of outcomes. Hip Int. 2014; 24(4):311–320.
Article22. Matharu GS, Eskelinen A, Judge A, Pandit HG, Murray DW. Revision surgery of metal-on-metal hip arthroplasties for adverse reactions to metal debris. Acta Orthop. 2018; 89(3):278–288.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metal-on-Metal Bearing in Total Hip Arthroplasty
- Metal-on-metal Articulation of Total Hip Replacement
- Dissociation of Polyethylene liner in Metal backed Cup without Hip Dislocation History: A Case Report
- Total Hip Arthroplasty with Use of Metal-on-Metal Articulation
- International regulatory considerations pertaining to the development of stem cell-based veterinary medicinal products