J Adv Prosthodont.
2019 Oct;11(5):247-252. 10.4047/jap.2019.11.5.247.
The effect of periodontal and prosthodontic therapy on glycemic control in patients with diabetes
- Affiliations
-
- 1Department of Periodontology, Kyungpook National University School of Dentistry, Daegu, Republic of Korea. leejm@knu.ca.kr
- 2Department of Prosthodontics, Kyungpook National University School of Dentistry, Daegu, Republic of Korea.
- 3College of Pharmacy and Research Institute of Pharmaceutical Sciences, Kyungpook National University, Daegu, Republic of Korea. sangkyu@knu.ac.kr
- KMID: 2462107
- DOI: http://doi.org/10.4047/jap.2019.11.5.247
Abstract
- PURPOSE
To evaluate the effect of periodontal and prosthodontic therapy on glycated hemoglobin A(HbA1c) level in patients with diabetes.
MATERIALS AND METHODS
This is a retrospective study of 70 patients suffering from diabetes who visited the Kyungpook National University Hospital between January 2016 and May 2018. Patients underwent medical evaluation for their routine check-up, which includes laboratory test for HbA1c levels. Among the 70 patients, 35 patients also visited Kyungpook National University Dental Hospital during the same period to receive periodontal and prosthodontic therapy, while the other 35 patients did not receive such therapy. The HbA1c levels were compared before and after periodontal and prosthodontic therapy. Comparisons between groups and within groups were performed using independent t-test.
RESULTS
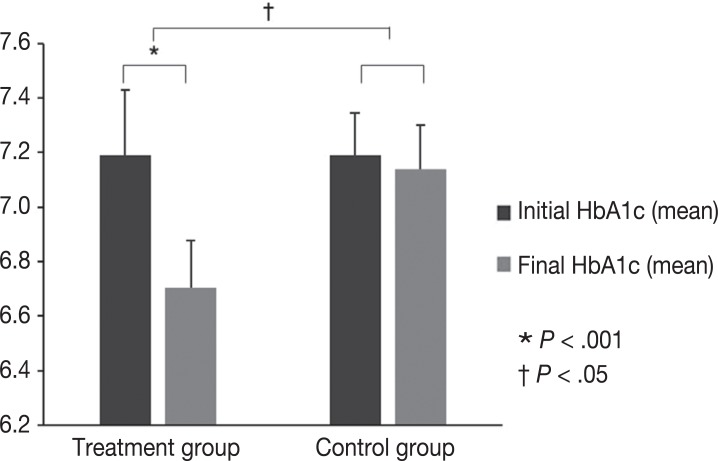
The HbA1c levels in the group who have received periodontal and prosthodontic therapy decreased from 7.2 to 6.7 (P=.001). The HbA1c levels in the control group decreased from 7.2 to 7.1 (P=.580). The difference in changes between the two patient groups was statistically significant (P=.011).
CONCLUSION
Periodontal and prosthodontic therapy can be effective on glycemic control in patients with diabetes.
MeSH Terms
Figure
Reference
-
1. Sonnenschein SK, Meyle J. Local inflammatory reactions in patients with diabetes and periodontitis. Periodontol 2000. 2015; 69:221–254. PMID: 26252411.
Article2. Mealey B. Diabetes and periodontal diseases. J Periodontol. 1999; 70:935–949.3. Xie W, Du L. Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diabetes Obes Metab. 2011; 13:289–301. PMID: 21205111.
Article4. Taiyeb-Ali TB, Raman RP, Vaithilingam RD. Relationship between periodontal disease and diabetes mellitus: an Asian perspective. Periodontol 2000. 2011; 56:258–268. PMID: 21501247.
Article5. Chávarry NG, Vettore MV, Sansone C, Sheiham A. The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysis. Oral Health Prev Dent. 2009; 7:107–127. PMID: 19583037.6. Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011; 7:738–748. PMID: 21709707.
Article7. Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991; 62:123–131. PMID: 2027060.
Article8. Lalla E, Cheng B, Lal S, Tucker S, Greenberg E, Goland R, Lamster IB. Periodontal changes in children and adolescents with diabetes: a case-control study. Diabetes Care. 2006; 29:295–299. PMID: 16443876.9. Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol. 1998; 69:76–83. PMID: 9527565.
Article10. Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Clin Periodontol. 2013; 40:S135–S152. PMID: 23627324.
Article11. Barros SP, Williams R, Offenbacher S, Morelli T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol 2000. 2016; 70:53–64. PMID: 26662482.
Article12. Salvi GE, Yalda B, Collins JG, Jones BH, Smith FW, Arnold RR, Offenbacher S. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol. 1997; 68:127–135. PMID: 9058329.
Article13. Wu T, Trevisan M, Genco RJ, Falkner KL, Dorn JP, Sempos CT. Examination of the relation between periodontal health status and cardiovascular risk factors: serum total and high density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. Am J Epidemiol. 2000; 151:273–282. PMID: 10670552.
Article14. Naguib G, Al-Mashat H, Desta T, Graves DT. Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J Invest Dermatol. 2004; 123:87–92. PMID: 15191547.
Article15. Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001; 72:1221–1227. PMID: 11577954.
Article16. Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000; 71:1528–1534. PMID: 11063384.
Article17. Mealey BL, Oates TW. American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. 2006; 77:1289–1303. PMID: 16881798.
Article18. Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001; 28:306–310. PMID: 11314885.
Article19. Li Q, Hao S, Fang J, Xie J, Kong XH, Yang JX. Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials. 2015; 16:291. PMID: 26137892.
Article20. Teshome A, Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health. 2016; 17:31. PMID: 27473177.
Article21. Mauri-Obradors E, Merlos A, Estrugo-Devesa A, Jané-Salas E, López-López J, Viñas M. Benefits of non-surgical periodontal treatment in patients with type 2 diabetes mellitus and chronic periodontitis: A randomized controlled trial. J Clin Periodontol. 2018; 45:345–353. PMID: 29265454.
Article22. Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, Mathur M, Montanya E, Shapira L, Tonetti M, Vegh D. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018; 45:138–149. PMID: 29280174.
Article23. Pretzl B, Kaltschmitt J, Kim TS, Reitmeir P, Eickholz P. Tooth loss after active periodontal therapy. 2: tooth-related factors. J Clin Periodontol. 2008; 35:175–182. PMID: 18199151.
Article24. Madianos PN, Koromantzos PA. An update of the evidence on the potential impact of periodontal therapy on diabetes outcomes. J Clin Periodontol. 2018; 45:188–195. PMID: 29277978.
Article25. Mauri-Obradors E, Jané-Salas E, Sabater-Recolons Mdel M, Vinas M, López-López J. Effect of nonsurgical periodontal treatment on glycosylated hemoglobin in diabetic patients: a systematic review. Odontology. 2015; 103:301–313. PMID: 25062756.
Article26. Wang TF, Jen IA, Chou C, Lei YP. Effects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: a meta-analysis. Medicine (Baltimore). 2014; 93:e292. PMID: 25526470.27. Moeintaghavi A, Arab HR, Bozorgnia Y, Kianoush K, Alizadeh M. Non-surgical periodontal therapy affects metabolic control in diabetics: a randomized controlled clinical trial. Aust Dent J. 2012; 57:31–37. PMID: 22369555.
Article28. Sun WL, Chen LL, Zhang SZ, Wu YM, Ren YZ, Qin GM. Inflammatory cytokines, adiponectin, insulin resistance and metabolic control after periodontal intervention in patients with type 2 diabetes and chronic periodontitis. Intern Med. 2011; 50:1569–1574. PMID: 21804283.
Article29. Iwamoto Y, Nishimura F, Nakagawa M, Sugimoto H, Shikata K, Makino H, Fukuda T, Tsuji T, Iwamoto M, Murayama Y. The effect of antimicrobial periodontal treatment on circulating tumor necrosis factor-alpha and glycated hemoglobin level in patients with type 2 diabetes. J Periodontol. 2001; 72:774–778. PMID: 11453240.
Article30. Matsuda Y, Minagawa T, Okui T, Yamazaki K. Resveratrol suppresses the alveolar bone resorption induced by artificial trauma from occlusion in mice. Oral Dis. 2018; 24:412–421. PMID: 28944599.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of scaling and root planing combined with systemic doxycycline therapy on glycemic control in diabetes mellitus subjects with chronic generalized periodontitis: a clinical study
- Glycemic Index Revisited
- Glycemic Control in Diabetic Patients with Diabetic Nephropathy
- Dental Health Care for Patients with Diabetes
- Subjective Assessment of Diabetes Self-Care Correlates with Perceived Glycemic Control but not with Actual Glycemic Control