Korean J Radiol.
2019 Oct;20(10):1422-1430. 10.3348/kjr.2019.0286.
Computed Tomography Pulmonary Vascular Volume Ratio Can Be Used to Evaluate the Effectiveness of Pulmonary Angioplasty in Peripheral Pulmonary Artery Stenosis
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. ghw68@hanmail.net
- KMID: 2459161
- DOI: http://doi.org/10.3348/kjr.2019.0286
Abstract
OBJECTIVE
To explore whether computed tomography (CT) pulmonary vascular volume ratio can be used to evaluate the effectiveness of pulmonary artery angioplasty in patients with peripheral pulmonary artery stenosis.
MATERIALS AND METHODS
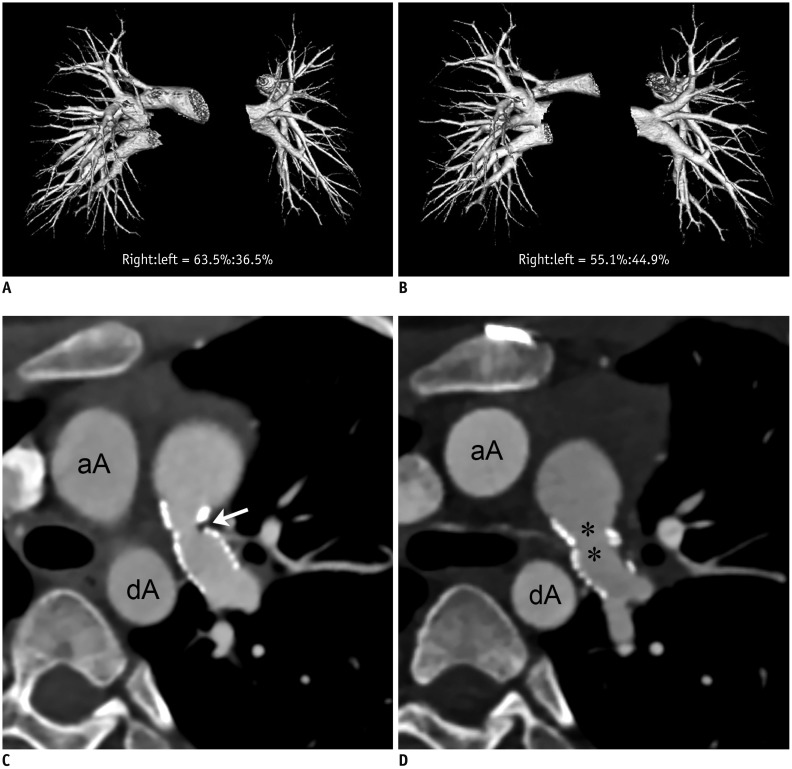
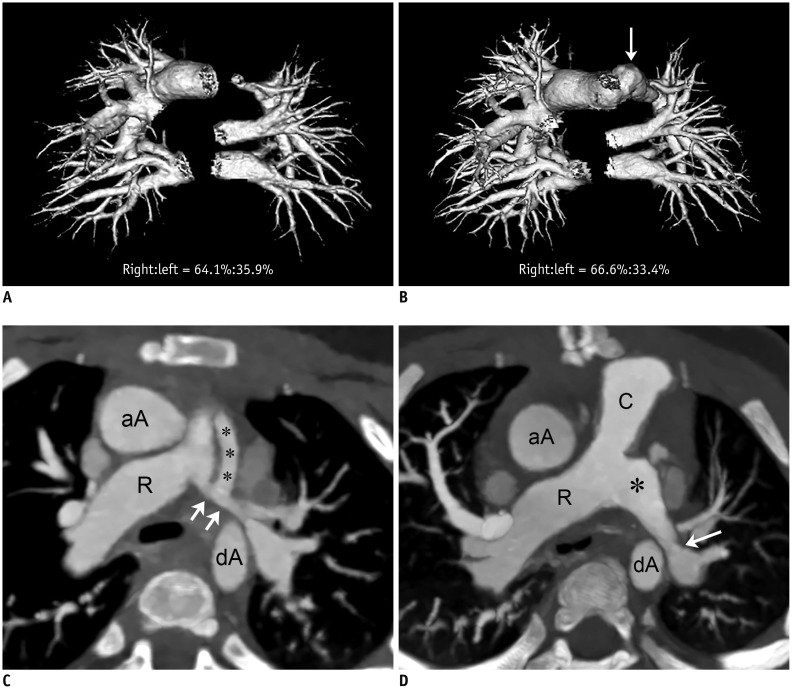
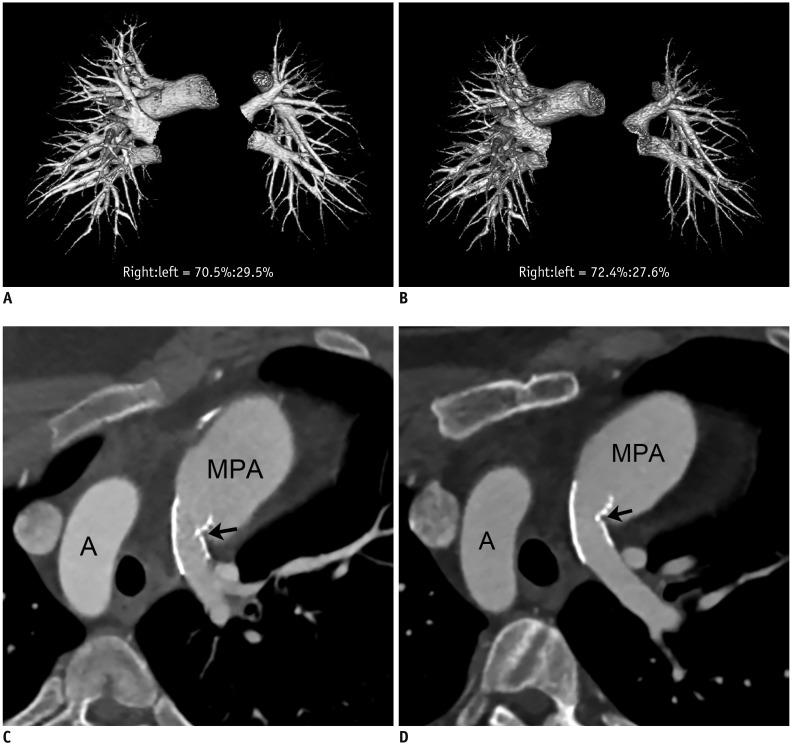
Changes in CT pulmonary vascular volume ratio between serial cardiothoracic CT examinations were calculated in 38 patients. Fifteen patients underwent interim pulmonary artery angioplasty (group 1), while 23 did not (group 2). According to the effectiveness of pulmonary artery angioplasty, patients in group 1 were further divided into group 1A (improved or aggravated) and group 1B (ineffective). Changes in the pulmonary vascular volume percentages among the three groups (group 1A, group 1B, and group 2) on serial CT examinations were compared.
RESULTS
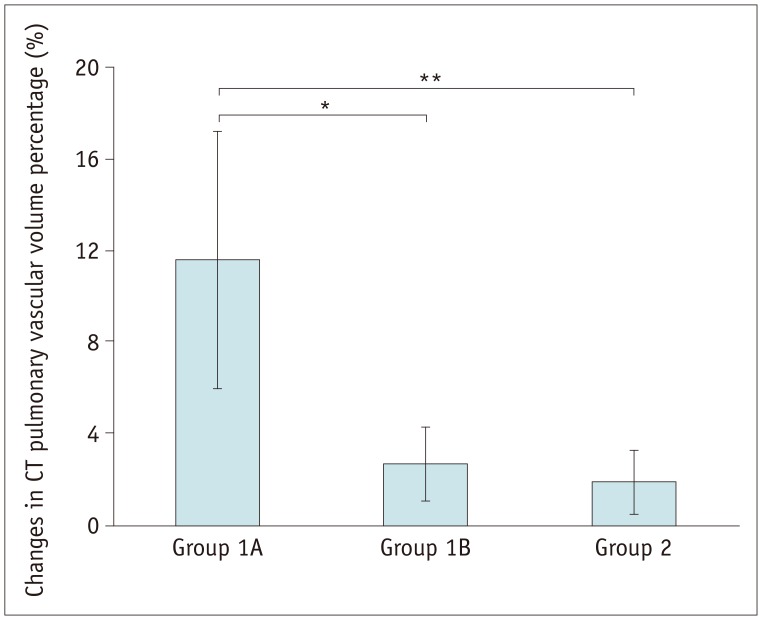
Pulmonary artery angioplasty on serial CT examinations was successful in seven patients, ineffective in seven patients, and aggravated in one patient. As a result, eight patients were included in group 1A and seven were included in group 1B. Changes in the CT pulmonary vascular volume percentages in group 1A were statistically significantly greater than those in group 1B (11.6 ± 5.6% vs. 2.7 ± 1.6%, p < 0.003) and group 2 (11.6 ± 5.6% vs. 1.9 ± 1.4%, p < 0.002), while no statistically significant difference was found between group 1B and group 2 (2.7 ± 1.6% vs. 1.9 ± 1.4%, p > 0.1).
CONCLUSION
CT pulmonary vascular volume ratio can be used to evaluate the effectiveness of pulmonary artery angioplasty in patients with peripheral pulmonary artery stenosis.
Keyword
Figure
Reference
-
1. Vida VL, Rito ML, Zucchetta F, Biffanti R, Padalino MA, Milanesi O, et al. Pulmonary artery branch stenosis in patients with congenital heart disease. J Card Surg. 2013; 28:439–445. PMID: 23718834.
Article2. Trivedi KR, Benson LN. Interventional strategies in the management of peripheral pulmonary artery stenosis. J Interv Cardiol. 2003; 16:171–188. PMID: 12768922.
Article3. Al-Khaldi A, Tamimi O. Surgical reconstruction of peripheral pulmonary arteries: strategies, outcomes, and new classification. Ann Thorac Surg. 2015; 100:623–630. PMID: 26138762.
Article4. Chien KJ, Huang HW, Huang TC, Lee CL, Weng KP, Lin CC, et al. Assessment of branch pulmonary artery stenosis in children after repair of tetralogy of Fallot using lung perfusion scintigraphy comparison with echocardiography. Ann Nucl Med. 2016; 30:49–59. PMID: 26493388.
Article5. Roman KS, Kellenberger CJ, Farooq S, MacGowan CK, Gilday DL, Yoo SJ. Comparative imaging of differential pulmonary blood flow in patients with congenital heart disease: magnetic resonance imaging versus lung perfusion scintigraphy. Pediatr Radiol. 2005; 35:295–301. PMID: 15490145.
Article6. Goo HW, Park SH. Pulmonary vascular volume ratio measured by cardiac computed tomography in children and young adults with congenital heart disease: comparison with lung perfusion scintigraphy. Pediatr Radiol. 2017; 47:1580–1587. PMID: 28646273.
Article7. Goo HW. Computed tomography pulmonary vascular volume ratio in children and young adults with congenital heart disease: the effect of cardiac phase. Pediatr Radiol. 2018; 48:915–922. PMID: 29572746.
Article8. Goo HW, Suh DS. Tube current reduction in pediatric non-ECG-gated heart CT by combined tube current modulation. Pediatr Radiol. 2006; 36:344–351. PMID: 16501970.
Article9. Goo HW. State-of-the-art CT imaging techniques for congenital heart disease. Korean J Radiol. 2010; 11:4–18. PMID: 20046490.
Article10. Goo HW. Individualized volume CT dose index determined by cross-sectional area and mean density of the body to achieve uniform image noise of contrast-enhanced pediatric chest CT obtained at variable kV levels and with combined tube current modulation. Pediatr Radiol. 2011; 41:839–847. PMID: 21656275.
Article11. Goo HW. Is it better to enter a volume CT dose index value before or after scan range adjustment for radiation dose optimization of pediatric cardiothoracic CT with tube current modulation? Korean J Radiol. 2018; 19:692–703. PMID: 29962875.
Article12. Goo HW. Comparison of chest pain protocols for electrocardiography-gated dual-source cardiothoracic CT in children and adults: the effect of tube current saturation on radiation dose reduction. Korean J Radiol. 2018; 19:23–31. PMID: 29353996.
Article13. Hong SH, Goo HW, Maeda E, Choo KS, Tsai IC. Asian Society of Cardiovascular Imaging Congenital Heart Disease Study Group. User-friendly vendor-specific guideline for pediatric cardiothoracic computed tomography provided by the Asian Society of Cardiovascular Imaging Congenital Heart Disease Study Group: part 1. Imaging techniques. Korean J Radiol. 2019; 20:190–204. PMID: 30672159.
Article14. Tricarico F, Hlavacek AM, Schoepf UJ, Ebersberger U, Nance JW Jr, Vliegenthart R, et al. Cardiovascular CT angiography in neonates and children: image quality and potential for radiation dose reduction with iterative image reconstruction techniques. Eur Radiol. 2013; 23:1306–1315. PMID: 23207869.
Article15. Goo HW. Combined prospectively electrocardiography- and respiratory-triggered sequential cardiac computed tomography in free-breathing children: success rate and image quality. Pediatr Radiol. 2018; 48:923–931. PMID: 29589058.
Article16. Goo HW. CT radiation dose optimization and estimation: an update for radiologists. Korean J Radiol. 2012; 13:1–11. PMID: 22247630.
Article17. Rothman A, Perry SB, Keane JF, Lock JE. Early results and follow-up of balloon angioplasty for branch pulmonary artery stenoses. J Am Coll Cardiol. 1990; 15:1109–1117. PMID: 2138184.
Article18. Hoshina M, Tomita H, Kimura K, Ono Y, Yagihara T, Echigo S. Factors determining peripheral pulmonary artery stenosis remodeling in children after percutaneous transluminal balloon angioplasty. Circ J. 2002; 66:345–348. PMID: 11954947.
Article19. Lock JE, Castaneda-Zuniga WR, Fuhrman BP, Bass JL. Balloon dilation angioplasty of hypoplastic and stenotic pulmonary arteries. Circulation. 1983; 67:962–967. PMID: 6219829.
Article20. Ring JC, Bass JL, Marvin W, Fuhrman BP, Kulik TJ, Foker JE, et al. Management of congenital stenosis of a branch pulmonary artery with balloon dilation angioplasty. Report of 52 procedures. J Thorac Cardiovasc Surg. 1985; 90:35–34. PMID: 3159939.21. Cunningham JW, McElhinney DB, Gauvreau K, Bergersen L, Lacro RV, Marshall AC, et al. Outcomes after primary transcatheter therapy in infants and young children with severe bilateral peripheral pulmonary artery stenosis. Circ Cardiovasc Interv. 2013; 6:460–467. PMID: 23941859.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Severe Pulmonary Hypertension Caused by Pulmonary Artery Stenosis in a Patient with Behcet's Disease Treated by Balloon Angioplasty
- Clinical application of pulmonary venous wedge angiogram
- Echogenic Mass Lesion within the Main Pulmonary Artery in a Neonate
- Absent Pulmonary Valve with Intact Ventricular Septum, PDA, ASD
- Spectrum of Multi-Detector Computed Tomography Findings that Alter Pulmonary Artery Diameters in Adults