Korean Circ J.
2019 Aug;49(8):678-690. 10.4070/kcj.2019.0163.
Pediatric Ventricular Assist Device
- Affiliations
-
- 1Division of Cardiovascular Surgery, Department of Thoracic and Cardiovascular Surgery, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea. hank@yuhs.ac
- KMID: 2456863
- DOI: http://doi.org/10.4070/kcj.2019.0163
Abstract
- There have been great advances in ventricular assist device (VAD) treatment for pediatric patients with advanced heart failure. VAD support provides more time for the patient in the heart transplant waiting list. Augmented cardiac output improves heart failure symptoms, end-organ function, and general condition, and consequently provides beneficial effects on post-transplant outcomes. Miniaturized continuous flow devices are more widely adopted for pediatric patient with promising results. For infants and small children, still paracorporeal pulsatile device is the only option for long-term support. Younger age, congenital heart disease, biventricular support, patient's status and end-organ dysfunction at the time of implantation are risks for poor outcomes. Patient selection, timing of implantation, and selection of device for each patient are critical for optimal clinical outcomes.
Keyword
MeSH Terms
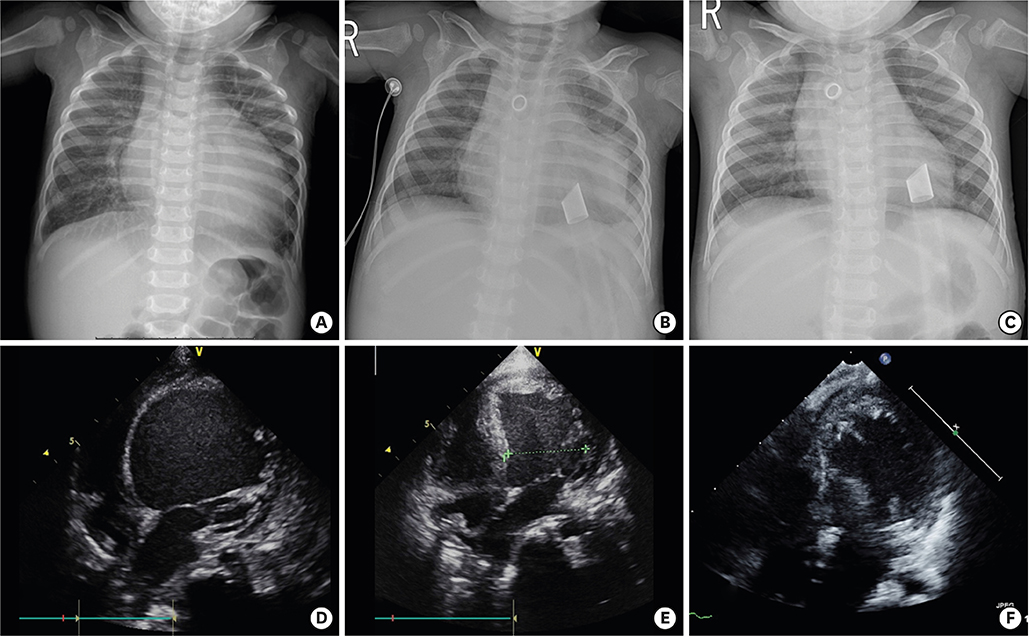
Figure
Cited by 1 articles
-
Pediatric Acute Myocarditis: Current Approach to Diagnosis and Treatment
Soo-Jin Kim
Korean Circ J. 2020;50(11):1023-1025. doi: 10.4070/kcj.2020.0389.
Reference
-
1. Rossano JW, Kim JJ, Decker JA, et al. Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: a population-based study. J Card Fail. 2012; 18:459–470.
Article2. Fraser CD Jr, Jaquiss RD, Rosenthal DN, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med. 2012; 367:532–541.
Article3. Rossano JW, Jang GY. Pediatric heart failure: current state and future possibilities. Korean Circ J. 2015; 45:1–8.
Article4. Peura JL, Colvin-Adams M, Francis GS, et al. Recommendations for the use of mechanical circulatory support: device strategies and patient selection: a scientific statement from the American Heart Association. Circulation. 2012; 126:2648–2667.5. Almond CS, Morales DL, Blackstone EH, et al. Berlin Heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation. 2013; 127:1702–1711.6. Hetzer R, Kaufmann F, Delmo Walter EM. Paediatric mechanical circulatory support with Berlin Heart EXCOR: development and outcome of a 23-year experience. Eur J Cardiothorac Surg. 2016; 50:203–210.
Article7. Reddy SL, Hasan A, Hamilton LR, et al. Mechanical versus medical bridge to transplantation in children. What is the best timing for mechanical bridge? Eur J Cardiothorac Surg. 2004; 25:605–609.
Article8. Wilmot I, Lorts A, Morales D. Pediatric mechanical circulatory support. Korean J Thorac Cardiovasc Surg. 2013; 46:391–401.
Article9. Adachi I, Burki S, Zafar F, Morales DL. Pediatric ventricular assist devices. J Thorac Dis. 2015; 7:2194–2202.10. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001; 345:1435–1443.11. Salzberg SP, Lachat ML, von Harbou K, Zünd G, Turina MI. Normalization of high pulmonary vascular resistance with LVAD support in heart transplantation candidates. Eur J Cardiothorac Surg. 2005; 27:222–225.
Article12. Yilmaz B, Zuckerman WA, Lee TM, et al. Left ventricular assist device to avoid heart-lung transplant in an adolescent with dilated cardiomyopathy and severely elevated pulmonary vascular resistance. Pediatr Transplant. 2013; 17:E113–E116.
Article13. Kumarasinghe G, Jain P, Jabbour A, et al. Comparison of continuous-flow ventricular assist device therapy with intensive medical therapy in fixed pulmonary hypertension secondary to advanced left heart failure. ESC Heart Fail. 2018; 5:695–702.
Article14. Iodice F, Testa G, Averardi M, Brancaccio G, Amodeo A, Cogo P. Implantation of a left ventricular assist device as a destination therapy in Duchenne muscular dystrophy patients with end stage cardiac failure: management and lessons learned. Neuromuscul Disord. 2015; 25:19–23.
Article15. Ryan TD, Jefferies JL, Sawnani H, et al. Implantation of the HeartMate II and HeartWare left ventricular assist devices in patients with duchenne muscular dystrophy: lessons learned from the first applications. ASAIO J. 2014; 60:246–248.16. Pearce FB, Kirklin JK, Holman WL, Barrett CS, Romp RL, Lau YR. Successful cardiac transplant after Berlin Heart bridge in a single ventricle heart: use of aortopulmonary shunt as a supplementary source of pulmonary blood flow. J Thorac Cardiovasc Surg. 2009; 137:e40–e42.
Article17. Lal AK, Chen S, Maeda K, et al. Successful bridge to transplant with a continuous flow ventricular assist device in a single ventricle patient with an aortopulmonary shunt. ASAIO J. 2014; 60:119–121.
Article18. Weinstein S, Bello R, Pizarro C, et al. The use of the Berlin Heart EXCOR in patients with functional single ventricle. J Thorac Cardiovasc Surg. 2014; 147:697–704.
Article19. Adachi I, Jeewa A, Burki S, McKenzie ED, Fraser CD Jr. Outpatient management of a child with bidirectional Glenn shunts supported with implantable continuous-flow ventricular assist device. J Heart Lung Transplant. 2016; 35:688–690.
Article20. Peng DM, Huebler M, Eghtesady P. Support strategies for single ventricle patients. In : Lorts A, Schweiger M, Conway J, Kirklin JK, editors. ISHLT Monograph Series: Pediatric Ventricular Assist Devices. Birmingham: UAB Printing;2017. p. 76–88.21. Valeske K, Yerebakan C, Mueller M, Akintuerk H. Urgent implantation of the Berlin Heart Excor biventricular assist device as a total artificial heart in a patient with single ventricle circulation. J Thorac Cardiovasc Surg. 2014; 147:1712–1714.22. Cedars A, Vanderpluym C, Koehl D, Cantor R, Kutty S, Kirklin JK. An Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of hospitalization, functional status, and mortality after mechanical circulatory support in adults with congenital heart disease. J Heart Lung Transplant. 2018; 37:619–630.
Article23. Morales DL, Adachi I, Heinle JS, Fraser CD Jr. A new era: use of an intracorporeal systemic ventricular assist device to support a patient with a failing Fontan circulation. J Thorac Cardiovasc Surg. 2011; 142:e138–e140.
Article24. Horne D, Conway J, Rebeyka IM, Buchholz H. Mechanical circulatory support in univentricular hearts: current management. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2015; 18:17–24.
Article25. Imielski BR, Niebler RA, Kindel SJ, Woods RK. HeartWare ventricular assist device implantation in patients with Fontan physiology. Artif Organs. 2017; 41:40–46.
Article26. Schmitt B, Steendijk P, Ovroutski S, et al. Pulmonary vascular resistance, collateral flow, and ventricular function in patients with a Fontan circulation at rest and during dobutamine stress. Circ Cardiovasc Imaging. 2010; 3:623–631.
Article27. Prêtre R, Häussler A, Bettex D, Genoni M. Right-sided univentricular cardiac assistance in a failing Fontan circulation. Ann Thorac Surg. 2008; 86:1018–1020.
Article28. Morales DL, Khan MS, Gottlieb EA, Krishnamurthy R, Dreyer WJ, Adachi I. Implantation of total artificial heart in congenital heart disease. Semin Thorac Cardiovasc Surg. 2012; 24:142–143.
Article29. Rossano JW, Goldberg DJ, Fuller S, Ravishankar C, Montenegro LM, Gaynor JW. Successful use of the total artificial heart in the failing Fontan circulation. Ann Thorac Surg. 2014; 97:1438–1440.
Article30. Park SJ, Liao KK, Segurola R, Madhu KP, Miller LW. Management of aortic insufficiency in patients with left ventricular assist devices: a simple coaptation stitch method (Park's stitch). J Thorac Cardiovasc Surg. 2004; 127:264–266.
Article31. Hetzer R, Potapov EV, Stiller B, et al. Improvement in survival after mechanical circulatory support with pneumatic pulsatile ventricular assist devices in pediatric patients. Ann Thorac Surg. 2006; 82:917–924.
Article32. Topilsky Y, Pereira NL, Shah DK, et al. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ Heart Fail. 2011; 4:266–275.
Article33. Tunuguntla H, Denfield SW, McKenzie ED, Adachi I. Mitral valve replacement for inflow obstruction of left ventricular assist device in a child with restrictive cardiomyopathy. J Thorac Cardiovasc Surg. 2016; 151:e11–3.
Article34. Blume ED, Rosenthal DN, Rossano JW, et al. Outcomes of children implanted with ventricular assist devices in the United States: first analysis of the pediatric interagency registry for mechanical circulatory support (PediMACS). J Heart Lung Transplant. 2016; 35:578–584.
Article35. Park YH. Current mechanical circulatory support devices for end stage heart failure. Korean Circ J. 2009; 39:1–10.
Article36. Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009; 361:2241–2251.
Article37. Adachi I, Schweiger M, Bruki S, Ozbaran M. Ventricular assist devices – durable device (continuous flow). In : Lorts A, Schweiger M, Conway J, Kirklin JK, editors. ISHLT Monograph Series: Pediatric Ventricular Assist Devices. Birmingham: UAB Printing;2017. p. 46–61.38. Miera O, Potapov EV, Redlin M, et al. First experiences with the HeartWare ventricular assist system in children. Ann Thorac Surg. 2011; 91:1256–1260.
Article39. Padalino MA, Bottio T, Tarzia V, et al. HeartWare ventricular assist device as bridge to transplant in children and adolescents. Artif Organs. 2014; 38:418–422.
Article40. Conway J, Miera O, Adachi I, et al. Worldwide experience of a durable centrifugal flow pump in pediatric patients. Semin Thorac Cardiovasc Surg. 2018; 30:327–335.
Article41. Copeland JG, Smith RG, Arabia FA, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004; 351:859–867.
Article42. Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol. 2015; 66:2663–2674.43. Birks EJ. Molecular changes after left ventricular assist device support for heart failure. Circ Res. 2013; 113:777–791.
Article44. Cruz DN, Schmidt-Ott KM, Vescovo G, et al. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: workgroup statements from the eleventh consensus conference of the acute dialysis quality initiative (ADQI). Contrib Nephrol. 2013; 182:117–136.
Article45. Patel AM, Adeseun GA, Ahmed I, Mitter N, Rame JE, Rudnick MR. Renal failure in patients with left ventricular assist devices. Clin J Am Soc Nephrol. 2013; 8:484–496.
Article46. Tromp TR, de Jonge N, Joles JA. Left ventricular assist devices: a kidney's perspective. Heart Fail Rev. 2015; 20:519–532.
Article47. Nishi H, Toda K, Miyagawa S, et al. Prediction of outcome in patients with liver dysfunction after left ventricular assist device implantation. J Artif Organs. 2013; 16:404–410.
Article48. Sharma MS, Webber SA, Morell VO, et al. Ventricular assist device support in children and adolescents as a bridge to heart transplantation. Ann Thorac Surg. 2006; 82:926–932.
Article49. Stiller B, Hetzer R, Weng Y, et al. Heart transplantation in children after mechanical circulatory support with pulsatile pneumatic assist device. J Heart Lung Transplant. 2003; 22:1201–1208.
Article50. Blume ED, Naftel DC, Bastardi HJ, et al. Outcomes of children bridged to heart transplantation with ventricular assist devices: a multi-institutional study. Circulation. 2006; 113:2313–2319.51. Karimova A, Pockett CR, Lasuen N, et al. Right ventricular dysfunction in children supported with pulsatile ventricular assist devices. J Thorac Cardiovasc Surg. 2014; 147:1691–1697.e1.
Article52. Stein ML, Robbins R, Sabati AA, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS)-defined morbidity and mortality associated with pediatric ventricular assist device support at a single US center: the Stanford experience. Circ Heart Fail. 2010; 3:682–688.53. Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010; 139:1316–1324.
Article54. Argenziano M, Choudhri AF, Moazami N, et al. Randomized, double-blind trial of inhaled nitric oxide in LVAD recipients with pulmonary hypertension. Ann Thorac Surg. 1998; 65:340–345.
Article55. Chang JC, Sawa Y, Ohtake S, et al. Hemodynamic effect of inhaled nitric oxide in dilated cardiomyopathy patients on LVAD support. ASAIO J. 1997; 43:M418–M421.
Article56. Lovich MA, Pezone MJ, Wakim MG, et al. Inhaled nitric oxide augments left ventricular assist device capacity by ameliorating secondary right ventricular failure. ASAIO J. 2015; 61:379–385.
Article57. Klodell CT Jr, Morey TE, Lobato EB, et al. Effect of sildenafil on pulmonary artery pressure, systemic pressure, and nitric oxide utilization in patients with left ventricular assist devices. Ann Thorac Surg. 2007; 83:68–71.
Article58. Stein ML, Dao DT, Doan LN, et al. Ventricular assist devices in a contemporary pediatric cohort: morbidity, functional recovery, and survival. J Heart Lung Transplant. 2016; 35:92–98.
Article59. Morales DL, Rossano JW, VanderPluym C, et al. Third annual pediatric interagency registry for mechanical circulatory support (PediMACS) report: preimplant characteristics and outcomes. Ann Thorac Surg. 2019; 107:993–1004.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The First Pediatric Heart Transplantation Bridged by a Durable Left Ventricular Assist Device in Korea
- Temporary Right Ventricular Assist Device Insertion via Left Thoracotomy after Left Ventricular Assist Device Implantation
- Development and animal study of a pediatric ventricular assist device
- Development and Animal Tests of Pneumatic Ventricular Assist Device
- Central-Approach Surgical Repair of Coarctation of the Aorta with a Back-up Left Ventricular Assist Device for an Infant Presenting with Severe Left Ventricular Dysfunction