Korean Circ J.
2019 Aug;49(8):657-677. 10.4070/kcj.2019.0188.
Clinical Pearls of Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Dongguk University Ilsan Hospital, Dongguk University School of Medicine, Goyang, Korea.
- 2Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. yanghyun.cho@samsung.com
- KMID: 2456862
- DOI: http://doi.org/10.4070/kcj.2019.0188
Abstract
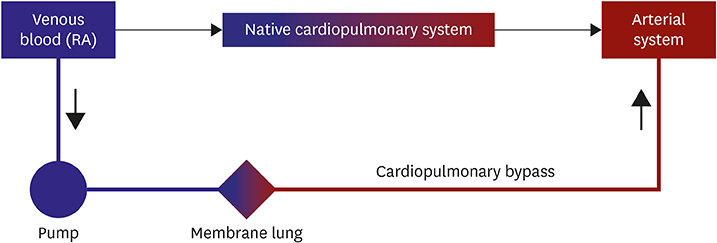
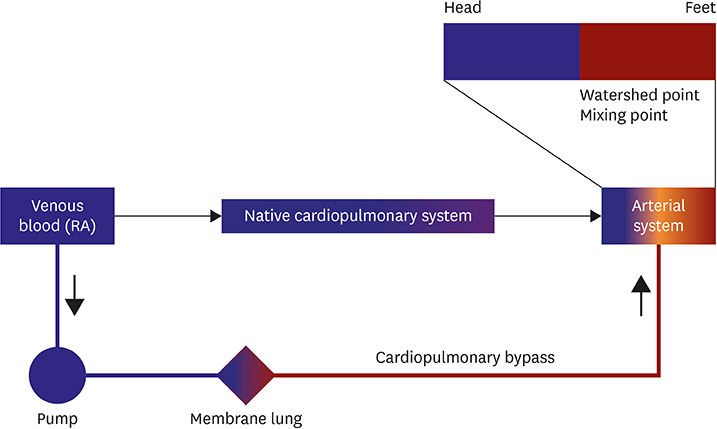
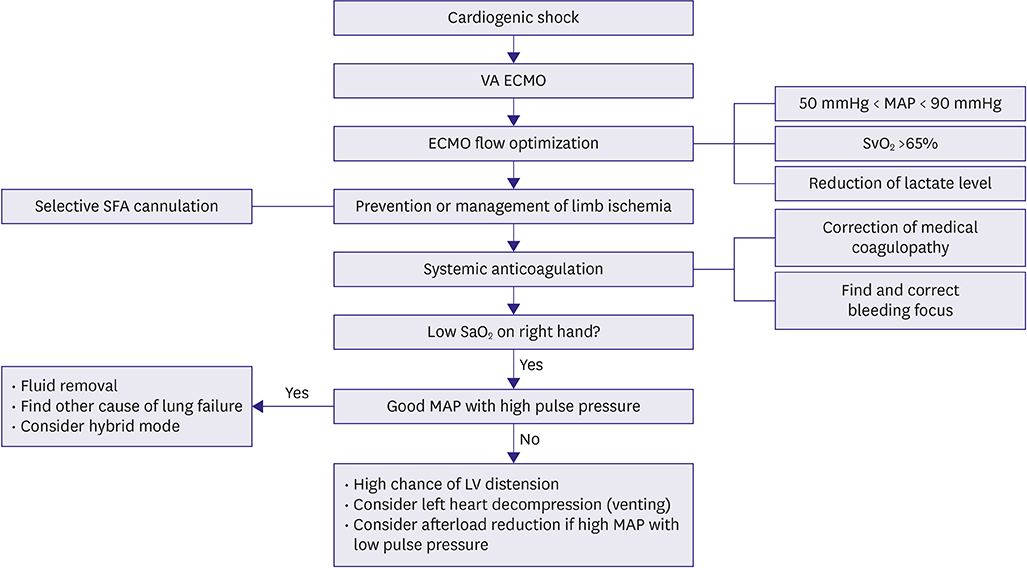
- Extracorporeal membrane oxygenation (ECMO) is a technique that uses a pump to drain blood from a body, circulate blood through a membrane lung, and return the oxygenated blood back into the body. Venoarterial (VA) ECMO is a simplified version of the heart-lung machine that assists native pulmonary and/or cardiac function. VA ECMO is composed of a drainage cannula in the venous system and a return cannula in the arterial system. Because VA ECMO can increase tissue perfusion by increasing the arterial blood flow, it is used to treat medically refractory cardiogenic shock or cardiac arrest. VA ECMO has a distinct physiology that is referred to as differential flows. It can cause several complications such as left ventricular distension with pulmonary edema, distal limb ischemia, bleeding, and thromboembolism. Physicians who are using this technology should be knowledgeable on the prevention and management of these complications. We review the basic physiology of VA ECMO, the mechanism of complications, and the simple management of VA ECMO.
MeSH Terms
Figure
Cited by 2 articles
-
Key Factors in Improving Clinical Outcomes in Patients with Cardiac Arrest Undergoing Extracorporeal Cardiopulmonary Resuscitation: a Multidisciplinary Team Approach
Jung-Joon Cha, Soon Jun Hong
Korean Circ J. 2021;51(11):919-921. doi: 10.4070/kcj.2021.0307.Left ventricle unloading during veno-arterial extracorporeal membrane oxygenation: review with updated evidence
Yongwhan Lim, Min Chul Kim, In-Seok Jeong
Acute Crit Care. 2024;39(4):473-487. doi: 10.4266/acc.2024.00801.
Reference
-
1. Fortenberry JD, Lorusso R. The history and development of extracorporeal support. In : Brogan TV, Lequier L, Lorusso R, MacLaren G, Peek G, editors. Extracorporeal Life Support: The ELSO Red Book. 5th ed. Ann Arbor (MI): Extracorporeal Life Support Organization;2017. p. 1–15.2. Toomasian JM, Vercaemst L, Bottrell S, Horton SB. The circuit. In : Brogan TV, Lequier L, Lorusso R, MacLaren G, Peek G, editors. Extracorporeal Life Support: The ELSO Red Book. 5th ed. Ann Arbor (MI): Extracorporeal Life Support Organization;2017. p. 49–80.3. Montoya JP, Shanley CJ, Merz SI, Bartlett RH. Plasma leakage through microporous membranes. Role of phospholipids. ASAIO J. 1992; 38:M399–M405.4. Thiara AP, Hoel TN, Kristiansen F, Karlsen HM, Fiane AE, Svennevig JL. Evaluation of oxygenators and centrifugal pumps for long-term pediatric extracorporeal membrane oxygenation. Perfusion. 2007; 22:323–326.
Article5. Bartlett RH, Conrad SA. The physiology of extracorporeal life support. In : Brogan TV, Lequier L, Lorusso R, MacLaren G, Peek G, editors. Extracorporeal Life Support: The ELSO Red Book. 5th ed. Ann Arbor (MI): Extracorporeal Life Support Organization;2017. p. 31–47.6. MacLaren G, Butt W. ECMO for septic shock. In : Brogan TV, Lequier L, Lorusso R, MacLaren G, Peek G, editors. Extracorporeal Life Support: The ELSO Red Book. 5th ed. Ann Arbor (MI): Extracorporeal Life Support Organization;2017. p. 613–626.7. Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol. 2015; 66:2663–2674.8. Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d' intervention. J Am Coll Cardiol. 2015; 65:e7–e26.9. Guglin M, Zucker MJ, Bazan VM, et al. Venoarterial ECMO for adults: JACC scientific expert panel. J Am Coll Cardiol. 2019; 73:698–716.10. Haft JW, Firmin R. Adult cardiac support. In : Annich GM, Lynch WR, MacLaren G, Wilson JM, Bartlett RH, editors. ECMO Extracorporeal Cardiopulmonary Support in Critical Care. 4th ed. Ann Arbor (MI): Extracorporeal Life Support Organization;2012. p. 323–330.11. Batista PM, Cavarocchi NC, Hirose H. Extracorporeal membranous oxygenation mimics aortic dissection on CAT scan. Ann Thorac Surg. 2013; 95:357.
Article12. Goslar T, Stankovic M, Ksela J. Contrast layering artefact mimicking aortic dissection in a patient on veno-arterial extracorporeal membrane oxygenation undergoing computed tomography scan. Interact Cardiovasc Thorac Surg. 2016; 22:507–509.
Article13. Sirol M, Sideris G, Deye N, Henry P, Baud F, Soyer P. A bizarre aortic dissection. Ann Thorac Surg. 2012; 93:2070.
Article14. Auzinger G, Best T, Vercueil A, Willars C, Wendon JA, Desai SR. Computed tomographic imaging in peripheral VA-ECMO: where has all the contrast gone? J Cardiothorac Vasc Anesth. 2014; 28:1307–1309.
Article15. Na SJ, Chung CR, Cho YH, et al. Vasoactive inotropic score as a predictor of mortality in adult patients with cardiogenic shock: medical therapy versus ECMO. Rev Esp Cardiol (Engl Ed). 2019; 72:40–47.
Article16. Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008; 372:554–561.
Article17. Shin TG, Choi JH, Jo IJ, et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: A comparison with conventional cardiopulmonary resuscitation. Crit Care Med. 2011; 39:1–7.
Article18. Huang SC, Wu ET, Chen YS, et al. Extracorporeal membrane oxygenation rescue for cardiopulmonary resuscitation in pediatric patients. Crit Care Med. 2008; 36:1607–1613.
Article19. Prodhan P, Fiser RT, Dyamenahalli U, et al. Outcomes after extracorporeal cardiopulmonary resuscitation (ECPR) following refractory pediatric cardiac arrest in the intensive care unit. Resuscitation. 2009; 80:1124–1129.
Article20. Sivarajan VB, Best D, Brizard CP, Shekerdemian LS, d'Udekem Y, Butt W. Duration of resuscitation prior to rescue extracorporeal membrane oxygenation impacts outcome in children with heart disease. Intensive Care Med. 2011; 37:853–860.
Article21. Jaski BE, Ortiz B, Alla KR, et al. A 20-year experience with urgent percutaneous cardiopulmonary bypass for salvage of potential survivors of refractory cardiovascular collapse. J Thorac Cardiovasc Surg. 2010; 139:753–757.e1-2.
Article22. Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015; 132:S444–S464.23. Lorusso R, Gelsomino S, Parise O, et al. Venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock in elderly patients: trends in application and outcome from the extracorporeal life support organization (ELSO) registry. Ann Thorac Surg. 2017; 104:62–69.
Article24. Šimek M, Hutyra M, Gwozdziewicz M, Fluger I, Steriovský A, Konečný J. The role of surgical embolectomy and extracorporeal membrane oxygen therapy in the treatment of massive pulmonary embolism - a review. Rozhl Chir. 2015; 94:103–110.25. Watanabe Y, Sakakura K, Akashi N, et al. Veno-arterial extracorporeal membrane oxygenation with conventional anticoagulation can be a best solution for shock due to massive PE. Int Heart J. 2017; 58:831–834.
Article26. Cho YH, Kim WS, Sung K, et al. Management of cardiac arrest caused by acute massive pulmonary thromboembolism: importance of percutaneous cardiopulmonary support. ASAIO J. 2014; 60:280–283.27. Swol J, Buchwald D, Ewers A, Schildhauer TA. Venoarterielle extrakorporale Membranoxygenierung (ECMO). Med Klin Intensivmed NotfMed. 2013; 108:63–68.
Article28. Yusuff HO, Zochios V, Vuylsteke A. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: a systematic review. Perfusion. 2015; 30:611–616.
Article29. Moon D, Lee SN, Yoo KD, Jo MS. Extracorporeal membrane oxygenation improved survival in patients with massive pulmonary embolism. Ann Saudi Med. 2018; 38:174–180.
Article30. Kafi A, Friedman O, Kim I. Use of low-dose thrombolytics for treatment of intracardiac thrombus and massive pulmonary embolus after aborted liver transplant leads to recovery of right ventricular function and redo liver transplantation. BMJ Case Rep. 2017; 2017:bcr-2017-219837.
Article31. Lauren Lindsey J, Jain R, Vachharajani V. Catheter directed thrombolysis combined with ECMO for massive pulmonary emboli. Respir Med Case Rep. 2018; 25:6–8.
Article32. Konstantinides SV. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014; 35:3145–3146.33. MacLaren G, Butt W, Best D, Donath S. Central extracorporeal membrane oxygenation for refractory pediatric septic shock. Pediatr Crit Care Med. 2011; 12:133–136.
Article34. Riera J, Argudo E, Ruiz-Rodríguez JC, Ferrer R. Extracorporeal membrane oxygenation for adults with refractory septic shock. ASAIO J. 2018; [Epub ahead of print].
Article35. Chvojka J, Martinkova V, Benes J, et al. Mechanical circulatory support in refractory vasodilatory septic shock: a randomized controlled porcine study. Shock. 2019; [Epub ahead of print].
Article36. Perdue SM, Poore BJ, Babu AN, Stribling WK. Successful use of extracorporeal membrane oxygenation support in severe septic shock with associated acute cardiomyopathy. J Card Surg. 2018; 33:50–52.
Article37. Asaki M, Masuda T, Miki Y. Veno-arterial extracorporeal membrane oxygenation for septic cardiomyopathy due to Legionella pneumonia after influenza virus infection. Case Rep Crit Care. 2018; 2018:6973197.38. Liu C, Zhu R, Zhou Z, et al. Sepsis-induced cardiomyopathy complicated with cardiogenic shock patients supported with extracorporeal membrane oxygenation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017; 29:1140–1143.39. Lees NJ, Rosenberg A, Hurtado-Doce AI, et al. Combination of ECMO and cytokine adsorption therapy for severe sepsis with cardiogenic shock and ARDS due to Panton-Valentine leukocidin-positive Staphylococcus aureus pneumonia and H1N1. J Artif Organs. 2016; 19:399–402.40. Kredel M, Kunzmann S, Schlegel PG, et al. Double peripheral venous and arterial cannulation for extracorporeal membrane oxygenation in combined septic and cardiogenic shock. Am J Case Rep. 2017; 18:723–727.
Article41. Pranikoff T, Hines MH. Vascular access for extracorporeal support. In : Annich GM, Lynch WR, MacLaren G, Wilson JM, Bartlett RH, editors. ECMO Extracorporeal Cardiopulmonary Support in Critical Care. 4th ed. Ann Arbor (MI): Extracorporeal Life Support Organization;2012. p. 133–147.42. Hysi I, Fabre O, Renaut C, Guesnier L. Extracorporeal membrane oxygenation with direct axillary artery perfusion. J Card Surg. 2014; 29:268–269.
Article43. Javidfar J, Brodie D, Costa J, et al. Subclavian artery cannulation for venoarterial extracorporeal membrane oxygenation. ASAIO J. 2012; 58:494–498.
Article44. Jayaraman AL, Cormican D, Shah P, Ramakrishna H. Cannulation strategies in adult veno-arterial and veno-venous extracorporeal membrane oxygenation: techniques, limitations, and special considerations. Ann Card Anaesth. 2017; 20:S11–8.
Article45. Napp LC, Kühn C, Hoeper MM, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol. 2016; 105:283–296.
Article46. Yang F, Hou D, Wang J, et al. Vascular complications in adult postcardiotomy cardiogenic shock patients receiving venoarterial extracorporeal membrane oxygenation. Ann Intensive Care. 2018; 8:72.
Article47. Aziz F, Brehm CE, El-Banyosy A, Han DC, Atnip RG, Reed AB. Arterial complications in patients undergoing extracorporeal membrane oxygenation via femoral cannulation. Ann Vasc Surg. 2014; 28:178–183.
Article48. Pozzi M, Koffel C, Djaref C, et al. High rate of arterial complications in patients supported with extracorporeal life support for drug intoxication-induced refractory cardiogenic shock or cardiac arrest. J Thorac Dis. 2017; 9:1988–1996.
Article49. Avalli L, Sangalli F, Migliari M, et al. Early vascular complications after percutaneous cannulation for extracorporeal membrane oxygenation for cardiac assist. Minerva Anestesiol. 2016; 82:36–43.50. Rao AS, Pellegrini RV, Speziali G, Marone LK. A novel percutaneous solution to limb ischemia due to arterial occlusion from a femoral artery ECMO cannula. J Endovasc Ther. 2010; 17:51–54.
Article51. Jang WJ, Cho YH, Park TK, et al. Fluoroscopy-guided simultaneous distal perfusion as a preventive strategy of limb ischemia in patients undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2018; 8:101.
Article52. Madershahian N, Nagib R, Wippermann J, Strauch J, Wahlers T. A simple technique of distal limb perfusion during prolonged femoro-femoral cannulation. J Card Surg. 2006; 21:168–169.
Article53. Yeo HJ, Yoon SH, Jeon D, et al. The utility of preemptive distal perfusion cannulation during peripheral venoarterial extracorporeal membrane oxygenation support. J Interv Cardiol. 2016; 29:431–436.
Article54. Lamb KM, Hirose H, Cavarocchi NC. Preparation and technical considerations for percutaneous cannulation for veno-arterial extracorporeal membrane oxygenation. J Card Surg. 2013; 28:190–192.
Article55. Keshavamurthy S, Shafii AE, Soltesz E. Spectroscopic limb monitoring in peripheral extracorporeal membrane oxygenation. Asian Cardiovasc Thorac Ann. 2015; 23:347–348.
Article56. Kapur NK, Esposito M. Hemodynamic support with percutaneous devices in patients with heart failure. Heart Fail Clin. 2015; 11:215–230.
Article57. Prasad A, Ghodsizad A, Brehm C, et al. Refractory pulmonary edema and upper body hypoxemia during veno-arterial extracorporeal membrane oxygenation-a case for atrial septostomy. Artif Organs. 2018; 42:664–669.
Article58. Schmack B, Seppelt P, Weymann A, et al. Extracorporeal life support with left ventricular decompression-improved survival in severe cardiogenic shock: results from a retrospective study. PeerJ. 2017; 5:e3813.
Article59. Alkhouli M, Narins CR, Lehoux J, Knight PA, Waits B, Ling FS. Percutaneous decompression of the left ventricle in cardiogenic shock patients on venoarterial extracorporeal membrane oxygenation. J Card Surg. 2016; 31:177–182.
Article60. Lee SI, Lee SY, Choi CH, Park KY, Park CH. Left heart decompression in acute complicated myocardial infarction during extracorporeal membrane oxygenation. J Intensive Care Med. 2017; 32:405–408.
Article61. Eastaugh LJ, Thiagarajan RR, Darst JR, McElhinney DB, Lock JE, Marshall AC. Percutaneous left atrial decompression in patients supported with extracorporeal membrane oxygenation for cardiac disease. Pediatr Crit Care Med. 2015; 16:59–65.
Article62. Cheung MM, Goldman AP, Shekerdemian LS, Brown KL, Cohen GA, Redington AN. Percutaneous left ventricular “vent” insertion for left heart decompression during extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2003; 4:447–449.
Article63. Dulnuan K, Guglin M, Zwischenberger J, Gurley J. Left atrial veno-arterial extracorporeal membrane oxygenation: percutaneous bi-atrial drainage to avoid pulmonary edema in patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2018; 71:A1358.
Article64. Baruteau AE, Barnetche T, Morin L, et al. Percutaneous balloon atrial septostomy on top of venoarterial extracorporeal membrane oxygenation results in safe and effective left heart decompression. Eur Heart J Acute Cardiovasc Care. 2018; 7:70–79.
Article65. Lin YN, Chen YH, Wang HJ, Hung JS, Chang KC, Lo PH. Atrial septostomy for left atrial decompression during extracorporeal membrane oxygenation by inoue balloon catheter. Circ J. 2017; 81:1419–1423.
Article66. Alhussein M, Osten M, Horlick E, et al. Percutaneous left atrial decompression in adults with refractory cardiogenic shock supported with veno-arterial extracorporeal membrane oxygenation. J Card Surg. 2017; 32:396–401.
Article67. Johnston TA, Jaggers J, McGovern JJ, O'Laughlin MP. Bedside transseptal balloon dilation atrial septostomy for decompression of the left heart during extracorporeal membrane oxygenation. Catheter Cardiovasc Interv. 1999; 46:197–199.
Article68. Weymann A, Schmack B, Sabashnikov A, et al. Central extracorporeal life support with left ventricular decompression for the treatment of refractory cardiogenic shock and lung failure. J Cardiothorac Surg. 2014; 9:60.
Article69. Keenan JE, Schechter MA, Bonadonna DK, et al. Early experience with a novel cannulation strategy for left ventricular decompression during nonpostcardiotomy venoarterial ECMO. ASAIO J. 2016; 62:e30–e34.
Article70. Kim C, Cho YH, Sung K, Yang JH. Transfromation of percutaneous extracorporeal life support to paracorporeal ventricular assist device: a case report. Korean J Thorac Cardiovasc Surg. 2014; 47:409–412.
Article71. Guirgis M, Kumar K, Menkis AH, Freed DH. Minimally invasive left-heart decompression during venoarterial extracorporeal membrane oxygenation: an alternative to a percutaneous approach. Interact Cardiovasc Thorac Surg. 2010; 10:672–674.
Article72. Centofanti P, Attisani M, La Torre M, et al. Left ventricular unloading during peripheral extracorporeal membrane oxygenator support: a bridge to life in profound cardiogenic shock. J Extra Corpor Technol. 2017; 49:201–205.73. Fumagalli R, Bombino M, Borelli M, et al. Percutaneous bridge to heart transplantation by venoarterial ECMO and transaortic left ventricular venting. Int J Artif Organs. 2004; 27:410–413.
Article74. Kim WH, Hong TH, Byun JH, et al. Flow rate through pigtail catheter used for left heart decompression in an artificial model of extracorporeal membrane oxygenation circuit. ASAIO J. 2017; 63:346–350.
Article75. Barbone A, Malvindi PG, Ferrara P, Tarelli G. Left ventricle unloading by percutaneous pigtail during extracorporeal membrane oxygenation. Interact Cardiovasc Thorac Surg. 2011; 13:293–295.
Article76. Loforte A, Baiocchi M, Gliozzi G, Coppola G, Di Bartolomeo R, Lorusso R. Percutaneous pulmonary artery venting via jugular vein while on peripheral extracorporeal membrane oxygenation running: a less invasive approach to provide full biventricular unloading. Ann Cardiothorac Surg. 2019; 8:163–166.
Article77. Avalli L, Maggioni E, Sangalli F, Favini G, Formica F, Fumagalli R. Percutaneous left-heart decompression during extracorporeal membrane oxygenation: an alternative to surgical and transeptal venting in adult patients. ASAIO J. 2011; 57:38–40.
Article78. Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005; 293:1653–1662.79. Coughlin MA, Bartlett RH. Anticoagulation for extracorporeal life support: direct thrombin inhibitors and heparin. ASAIO J. 2015; 61:652–655.80. Tay CK, Sung K, Cho YH. Clinical pearls in venovenous extracorporeal life support for adult respiratory failure. ASAIO J. 2018; 64:1–9.
Article81. Kim YS, Lee H, Yang JH, et al. Use of argatroban for extracorporeal life support in patients with nonheparin-induced thrombocytopenia: analysis of 10 consecutive patients. Medicine (Baltimore). 2018; 97:e13235.82. Beiderlinden M, Treschan T, Görlinger K, Peters J. Argatroban in extracorporeal membrane oxygenation. Artif Organs. 2007; 31:461–465.
Article83. Donker DW, Meuwese CL, Braithwaite SA, et al. Echocardiography in extracorporeal life support: a key player in procedural guidance, tailoring and monitoring. Perfusion. 2018; 33:31–41.
Article84. Hwang JW, Yang JH, Sung K, et al. Percutaneous removal using Perclose ProGlide closure devices versus surgical removal for weaning after percutaneous cannulation for venoarterial extracorporeal membrane oxygenation. J Vasc Surg. 2016; 63:998–1003.e1.
Article85. Aso S, Matsui H, Fushimi K, Yasunaga H. The effect of intraaortic balloon pumping under venoarterial extracorporeal membrane oxygenation on mortality of cardiogenic patients: an analysis using a nationwide inpatient database. Crit Care Med. 2016; 44:1974–1979.86. Park TK, Yang JH, Choi SH, et al. Clinical impact of intra-aortic balloon pump during extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock. BMC Anesthesiol. 2014; 14:27.
Article87. Tay CK, Yoo KH, Cho YH. Intraaortic balloon pulsation in peripheral venoarterial extracorporeal membrane oxygenation: more is not always better. Crit Care Med. 2016; 44:e1251.88. Karatolios K, Chatzis G, Markus B, Luesebrink U, Richter A, Schieffer B. Biventricular unloading in patients with refractory cardiogenic shock. Int J Cardiol. 2016; 222:247–252.
Article89. Cheng A, Swartz MF, Massey HT. Impella to unload the left ventricle during peripheral extracorporeal membrane oxygenation. ASAIO J. 2013; 59:533–536.
Article90. Narain S, Paparcuri G, Fuhrman TM, Silverman RB, Peruzzi WT. Novel combination of impella and extra corporeal membrane oxygenation as a bridge to full recovery in fulminant myocarditis. Case Rep Crit Care. 2012; 2012:459296.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Catastrophic catecholamine-induced cardiomyopathy rescued by extracorporeal membrane oxygenation in recurrent malignant pheochromocytoma
- A case of rescuing a patient with acute cardiovascular instability from sudden and massive intraoperative pulmonary thromboembolism by extracorporeal membrane oxygenation
- Mechanical Circulatory Support in the Cardiac Catheterization Laboratory for Cardiogenic Shock
- Successful Left-Heart Decompression during Extracorporeal Membrane Oxygenation in an Adult Patient by Percutaneous Transaortic Catheter Venting
- Acute fulminant myocarditis following influenza vaccination requiring extracorporeal membrane oxygenation