Korean J Physiol Pharmacol.
2019 Sep;23(5):329-334. 10.4196/kjpp.2019.23.5.329.
Effect of gemigliptin on cardiac ischemia/reperfusion and spontaneous hypertensive rat models
- Affiliations
-
- 1Predictive Model Research Center, Korea Institute of Toxicology, Daejeon 34114, Korea. baekeunbok@hanmail.net
- 2Center for Inflammation, Immunity & Infection, Institute for Biomedical Sciences, Georgia State University, Atlanta, GA 30303, USA.
- 3Corporate R&D, LG Chem, Ltd., Daejeon 34122, Korea. baekeunbok@hanmail.net
- 4Department of Human and Environmental Toxicology, University of Science and Technology, Daejeon 34113, Korea.
- KMID: 2455809
- DOI: http://doi.org/10.4196/kjpp.2019.23.5.329
Abstract
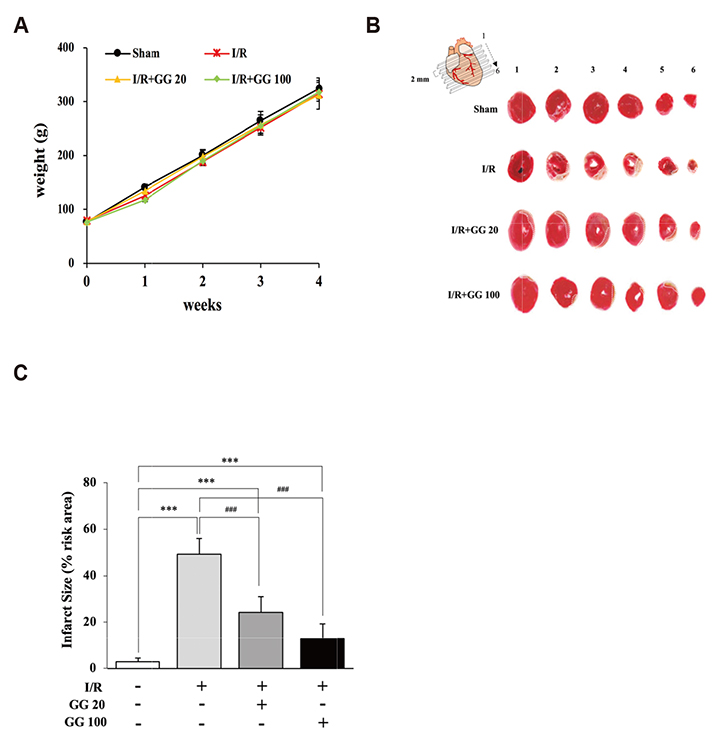
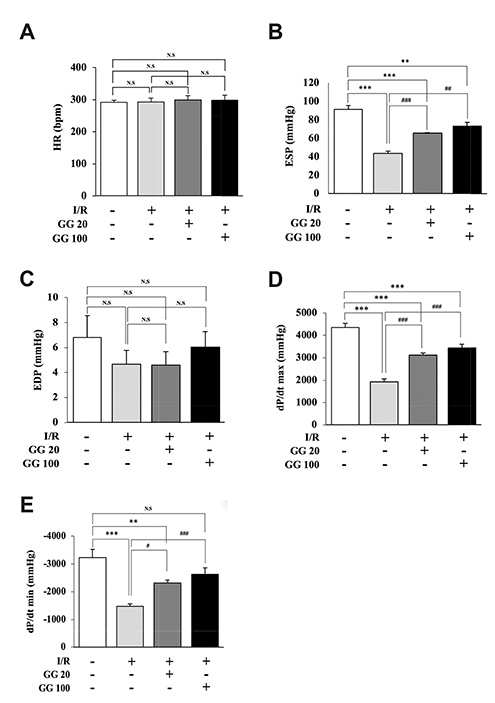
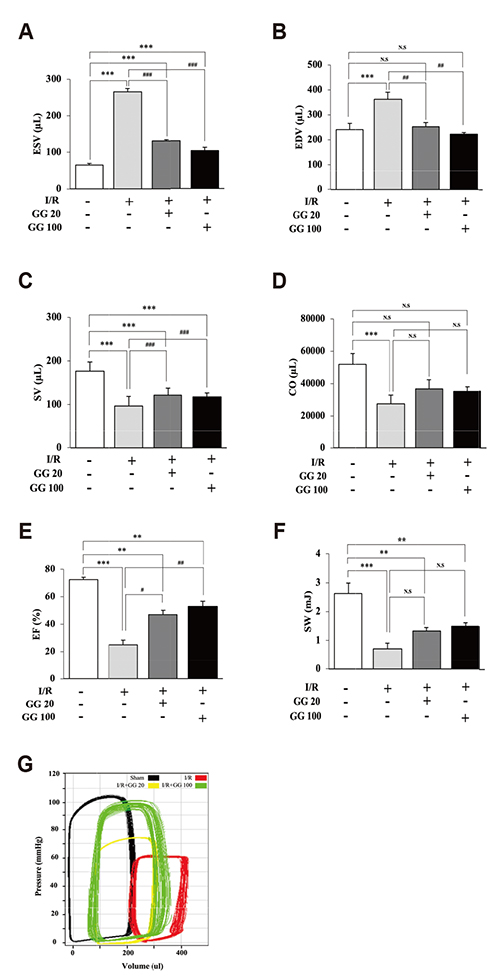
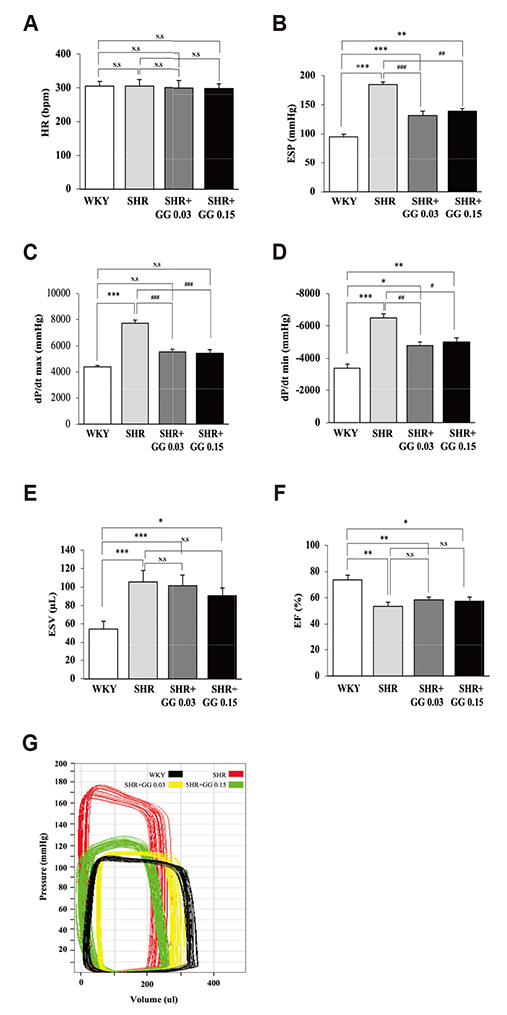
- Diabetes is associated with an increased risk of cardiovascular complications. Dipeptidyl peptidase-4 (DPP-IV) inhibitors are used clinically to reduce high blood glucose levels as an antidiabetic agent. However, the effect of the DPP-IV inhibitor gemigliptin on ischemia/reperfusion (I/R)-induced myocardial injury and hypertension is unknown. In this study, we assessed the effects and mechanisms of gemigliptin in rat models of myocardial I/R injury and spontaneous hypertension. Gemigliptin (20 and 100 mg/kg/d) or vehicle was administered intragastrically to Sprague-Dawley rats for 4 weeks before induction of I/R injury. Gemigliptin exerted a preventive effect on I/R injury by improving hemodynamic function and reducing infarct size compared to the vehicle control group. Moreover, administration of gemigliptin (0.03% and 0.15%) powder in food for 4 weeks reversed hypertrophy and improved diastolic function in spontaneously hypertensive rats. We report here a novel effect of the gemigliptin on I/R injury and hypertension.
Keyword
MeSH Terms
Figure
Reference
-
1. Daousi C, Casson IF, Gill GV, MacFarlane IA, Wilding JP, Pinkney JH. Prevalence of obesity in type 2 diabetes in secondary care: association with cardiovascular risk factors. Postgrad Med J. 2006; 82:280–284.
Article2. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998; 339:229–234.
Article3. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, et al. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011; 364:829–841.
Article4. Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014; 5:444–470.
Article5. Dardano A, Penno G, Del Prato S, Miccoli R. Optimal therapy of type 2 diabetes: a controversial challenge. Aging (Albany NY). 2014; 6:187–206.
Article6. Ahrén B. Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care. 2007; 30:1344–1350.
Article7. Lambeir AM, Durinx C, Scharpé S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003; 40:209–294.
Article8. Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol. 2011; 55:10–16.
Article9. Sauvé M, Ban K, Momen MA, Zhou YQ, Henkelman RM, Husain M, Drucker DJ. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010; 59:1063–1073.
Article10. Esposito G, Cappetta D, Russo R, Rivellino A, Ciuffreda LP, Roviezzo F, Piegari E, Berrino L, Rossi F, De Angelis A, Urbanek K. Sitagliptin reduces inflammation, fibrosis and preserves diastolic function in a rat model of heart failure with preserved ejection fraction. Br J Pharmacol. 2017; 174:4070–4086.
Article11. Koibuchi N, Hasegawa Y, Katayama T, Toyama K, Uekawa K, Sueta D, Kusaka H, Ma M, Nakagawa T, Lin B, Kim-Mitsuyama S. DPP-4 inhibitor linagliptin ameliorates cardiovascular injury in salt-sensitive hypertensive rats independently of blood glucose and blood pressure. Cardiovasc Diabetol. 2014; 13:157.
Article12. Ihara M, Asanuma H, Yamazaki S, Kato H, Asano Y, Shinozaki Y, Mori H, Minamino T, Asakura M, Sugimachi M, Mochizuki N, Kitakaze M. An interaction between glucagon-like peptide-1 and adenosine contributes to cardioprotection of a dipeptidyl peptidase 4 inhibitor from myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2015; 308:H1287–H1297.
Article13. Scheen AJ. GLP-1 receptor agonists and heart failure in diabetes. Diabetes Metab. 2017; 43 Suppl 1:2S13–2S19.
Article14. Powers SK, Smuder AJ, Kavazis AN, Quindry JC. Mechanisms of exercise-induced cardioprotection. Physiology (Bethesda). 2014; 29:27–38.
Article15. Kim SH, Jung E, Yoon MK, Kwon OH, Hwang DM, Kim DW, Kim J, Lee SM, Yim HJ. Pharmacological profiles of gemigliptin (LC15-0444), a novel dipeptidyl peptidase-4 inhibitor, in vitro and in vivo. Eur J Pharmacol. 2016; 788:54–64.16. Kim SH, Yoo JH, Lee WJ, Park CY. Gemigliptin: an update of its clinical use in the management of type 2 diabetes mellitus. Diabetes Metab J. 2016; 40:339–353.
Article17. Lim KS, Kim JR, Choi YJ, Shin KH, Kim KP, Hong JH, Cho JY, Shin HS, Yu KS, Shin SG, Kwon OH, Hwang DM, Kim JA, Jang IJ. Pharmacokinetics, pharmacodynamics, and tolerability of the dipeptidyl peptidase IV inhibitor LC15-0444 in healthy Korean men: a dose-block-randomized, double-blind, placebo-controlled, ascending single-dose, Phase I study. Clin Ther. 2008; 30:1817–1830.
Article18. Hausenloy DJ, Whittington HJ, Wynne AM, Begum SS, Theodorou L, Riksen N, Mocanu MM, Yellon DM. Dipeptidyl peptidase-4 inhibitors and GLP-1 reduce myocardial infarct size in a glucose-dependent manner. Cardiovasc Diabetol. 2013; 12:154.
Article19. Mandlem VVK, Annapurna A. Cardioprotective role of saxagliptin through antioxidant mechanism in experimental myocardial infarction in STZ induced diabetic rats. J Clin Exp Pharmacol. 2017; 7:1000233.20. Ku HC, Chen WP, Su MJ. DPP4 deficiency preserves cardiac function via GLP-1 signaling in rats subjected to myocardial ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol. 2011; 384:197–207.
Article21. Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, Lam KS, Chen ZY, Ng CF, Yao X, Huang Y. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012; 60:833–841.
Article22. Lee TI, Kao YH, Chen YC, Huang JH, Hsu MI, Chen YJ. The dipeptidyl peptidase-4 inhibitor-sitagliptin modulates calcium dysregulation, inflammation, and PPARs in hypertensive cardiomyocytes. Int J Cardiol. 2013; 168:5390–5395.
Article23. Giannocco G, Oliveira KC, Crajoinas RO, Venturini G, Salles TA, Fonseca-Alaniz MH, Maciel RM, Girardi AC. Dipeptidyl peptidase IV inhibition upregulates GLUT4 translocation and expression in heart and skeletal muscle of spontaneously hypertensive rats. Eur J Pharmacol. 2013; 698:74–86.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vitro Perfusion Studies on Coronary Function of Cardiac Ischemia-Reperfusion in Spontaneously Hypertensive Rat Heart
- The Effect of Melatonin on Biochemical Changes after Ischemia-Reperfusion Injury of Rat Skeletal Muscle
- The effects of hydrogen sulfide under sevoflurane administration against ischemia and reperfusion injury in isolated rat heart
- Effect of C1 esterase inhibitor on the cardiac dysfunction following ischemia and reperfusion in the isolated perfused rat heart
- Effect of Ischemic Preconditioning on Catecholamine Release from the Isolated, Ischemic Reperfused Hearts of Rats