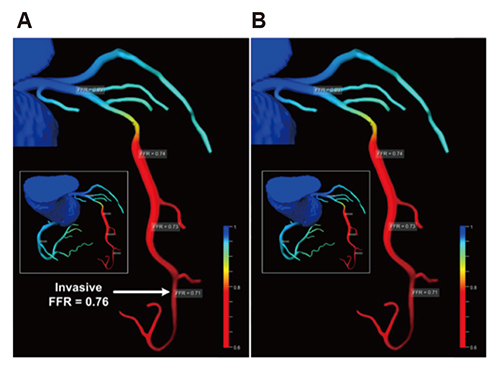
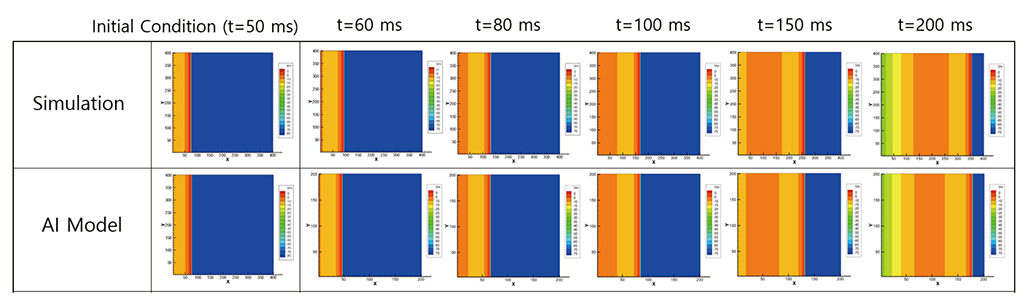
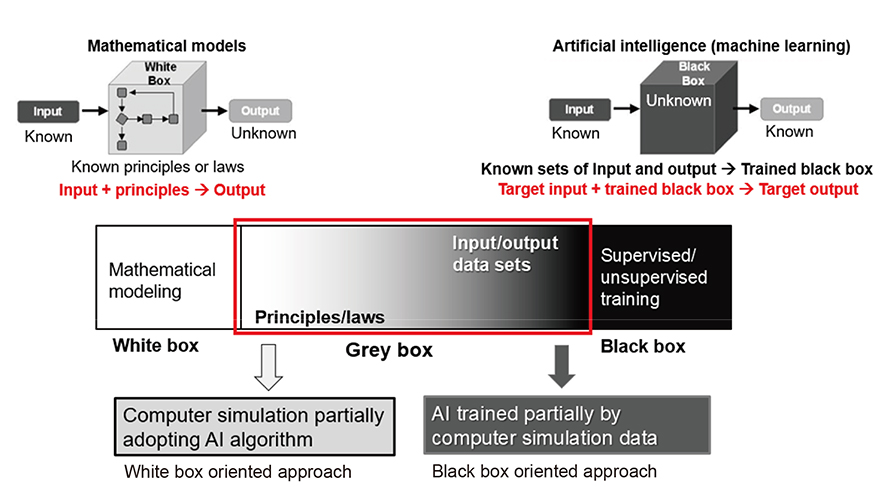
Korean J Physiol Pharmacol.
2019 Sep;23(5):305-310. 10.4196/kjpp.2019.23.5.305.
Toward a grey box approach for cardiovascular physiome
- Affiliations
-
- 1SiliconSapiens Inc., Seoul 06097, Korea. ebshim@kangwon.ac.kr
- 2Department of Physiology, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 3Department of Mechanical and Biomedical Engineering, Kangwon National University, Chuncheon 24341, Korea.
- KMID: 2455806
- DOI: http://doi.org/10.4196/kjpp.2019.23.5.305
Abstract
- The physiomic approach is now widely used in the diagnosis of cardiovascular diseases. There are two possible methods for cardiovascular physiome: the traditional mathematical model and the machine learning (ML) algorithm. ML is used in almost every area of society for various tasks formerly performed by humans. Specifically, various ML techniques in cardiovascular medicine are being developed and improved at unprecedented speed. The benefits of using ML for various tasks is that the inner working mechanism of the system does not need to be known, which can prove convenient in situations where determining the inner workings of the system can be difficult. The computation speed is also often higher than that of the traditional mathematical models. The limitations with ML are that it inherently leads to an approximation, and special care must be taken in cases where a high accuracy is required. Traditional mathematical models are, however, constructed based on underlying laws either proven or assumed. The results from the mathematical models are accurate as long as the model is. Combining the advantages of both the mathematical models and ML would increase both the accuracy and efficiency of the simulation for many problems. In this review, examples of cardiovascular physiome where approaches of mathematical modeling and ML can be combined are introduced.
MeSH Terms
Figure
Cited by 1 articles
-
Digital heart for life
Yin Hua Zhang
Korean J Physiol Pharmacol. 2019;23(5):291-293. doi: 10.4196/kjpp.2019.23.5.291.
Reference
-
1. Russell SJ, Norvig P. Artificial intelligence: a modern approach. 2nd ed. New Delhi: Prentice Hall;2003.2. Mitchell TM. Machine learning. New York: McGraw-Hill;1997.3. Schmidhuber J. Deep learning in neural networks: an overview. Neural Netw. 2015; 61:85–117.
Article4. Krizhevsky A, Sutskever I, Hinton GE. Pereira F, Burges CJC, Bottou L, Weinberger KQ. ImageNet classification with deep convolutional neural networks. In : NIPS'12 Proceedings of the 25th International Conference on neural information processing systems; New York: Curran Associates Inc;2012. p. 1097–1105.5. Goodfellow IJ, Pouget-Abadie J, Mirza M, Xu B, Warde-Farley D, Ozair S, Courville A, Bengio Y. In : Ghahramani Z, Welling M, Cortes C, Lawrence ND, Weinberger KQ, editors. Generative adversarial nets. NIPS'14 Proceedings of the 27th International Conference on neural information processing systems; Cambridge: MIT Press Cambridge;2014. p. 2672–2680.6. Ghahramani Z. Unsupervised learning. In : Bousquet O, von Luxburg U, Ratsch G, editors. Advanced lectures on machine learning. Berlin: New York: Springer;2004.7. Lundervold AS, Lundervold A. An overview of deep learning in medical imaging focusing on MRI. Z Med Phys. 2019; 29:102–127.
Article8. Ker J, Wang L, Rao J, Lim T. Deep learning applications in medical image analysis. IEEE Access. 2017; 6:9375–9389.
Article9. Liu S, Wang Y, Yang X, Lei B, Liu L, Li S, Ni D, Wang T. Deep learning in medical ultrasound analysis: a review. Engineering. 2019; 5:261–275.
Article10. Shen D, Wu G, Suk HI. Deep learning in medical image analysis. Annu Rev Biomed Eng. 2017; 19:221–248.
Article11. Maier A, Syben C, Lasser T, Riess C. A gentle introduction to deep learning in medical image processing. Z Med Phys. 2019; 29:86–101.
Article12. Bratt A, Kim J, Pollie M, Beecy AN, Tehrani NH, Codella N, Perez-Johnston R, Palumbo MC, Alakbarli J, Colizza W, Drexler IR, Azevedo CF, Kim RJ, Devereux RB, Weinsaft JW. Machine learning derived segmentation of phase velocity encoded cardiovascular magnetic resonance for fully automated aortic flow quantification. J Cardiovasc Magn Reson. 2019; 21:1.
Article13. Kurata A, Fukuyama N, Hirai K, Kawaguchi N, Tanabe Y, Okayama H, Shigemi S, Watanabe K, Uetani T, Ikeda S, Inaba S, Kido T, Itoh T, Mochizuki T. On-site computed tomography-derived fractional flow reserve using a machine-learning algorithm- clinical effectiveness in a retrospective multicenter cohort. Circ J. 2019; 83:1563–1571.14. Wang Z. Support vector machine learning-based cerebral blood flow quantification for arterial spin labeling MRI. Hum Brain Mapp. 2014; 35:2869–2875.
Article15. Jordanski M, Radovic M, Milosevic Z, Filipovic N, Obradovic Z. Machine learning approach for predicting wall shear distribution for abdominal aortic aneurysm and carotid bifurcation models. IEEE J Biomed Health Inform. 2018; 22:537–544.
Article16. Riordon J, Sovilj D, Sanner S, Sinton D, Young EWK. Deep learning with microfluidics for biotechnology. Trends Biotechnol. 2019; 37:310–324.
Article17. Itu L, Rapaka S, Passerini T, Georgescu B, Schwemmer C, Schoebinger M, Flohr T, Sharma P, Comaniciu D. A machine-learning approach for computation of fractional flow reserve from coronary computed tomography. J Appl Physiol (1985). 2016; 121:42–52.
Article18. McKinley R, Hung F, Wiest R, Liebeskind DS, Scalzo F. A machine learning approach to perfusion imaging with dynamic susceptibility contrast MR. Front Neurol. 2018; 9:717.
Article19. Cantwell CD, Mohamied Y, Tzortzis KN, Garasto S, Houston C, Chowdhury RA, Ng FS, Bharath AA, Peters NS. Rethinking multiscale cardiac electrophysiology with machine learning and predictive modelling. Comput Biol Med. 2019; 104:339–351.
Article20. Lyon A, Mincholé A, Martínez JP, Laguna P, Rodriguez B. Computational techniques for ECG analysis and interpretation in light of their contribution to medical advances. J R Soc Interface. 2018; 15:20170821.
Article21. Vamathevan J, Clark D, Czodrowski P, Dunham I, Ferran E, Lee G, Li B, Madabhushi A, Shah P, Spitzer M, Zhao S. Applications of machine learning in drug discovery and development. Nat Rev Drug Discov. 2019; 18:463–477.
Article22. Costabal FS, Matsuno K, Yao J, Perdikaris P, Kuhl E. Machine learning in drug development: characterizing the effect of 30 drugs on the QT interval using Gaussian process regression, sensitivity analysis, and uncertainty quantification. Comput Methods Appl Mech Eng. 2019; 348:313–333.23. Deist TM, Patti A, Wang Z, Krane D, Sorenson T, Craft D. Simulation assisted machine learning. Bioinformatics. 2019; DOI: 10.1093/bioinformatics/btz199. [Epub ahead of print].
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of a Grey Zone for HCV and HIV Immunoassays for Blood Donor Screening: Is It still Necessary?
- Thoraco-laparotomy approach to salvage a life-threatening cardiac box stab injury to the infecrior vena cava in Malaysia: a case report
- Long-Term Grey Matter Changes in First Episode Psychosis: A Systematic Review
- HBeAg-positive grey-zone patients: Treatment beyond guideline recommendations?
- Association of Temporal Lobe Atrophy and Psychosis in Patients with Alzheimer's Disease