Ann Clin Neurophysiol.
2019 Jul;21(2):79-86. 10.14253/acn.2019.21.2.79.
Pattern analysis of lower limb magnetic resonance images in Korean patients with distal myopathy
- Affiliations
-
- 1Department of Neurology, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea.
- 2Department of Neurology, Yonsei University College of Medicine, Seoul, Korea. ycchoi@yuhs.ac
- 3Department of Neurology, Mokdong Hospital, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 2454728
- DOI: http://doi.org/10.14253/acn.2019.21.2.79
Abstract
- BACKGROUND
Magnetic resonance (MR) images are useful for diagnosing myopathy. The purpose of this study was to determine the usefulness of lower-limb MR images in Korean patients with distal myopathy.
METHODS
We reviewed medical records in the myopathy database from January 2002 to October 2016. We selected 21 patients from 91 unrelated families with distal myopathy: four with GNE myopathy, 11 with dysferlinopathy, and six with ADSSL1 myopathy.
RESULTS
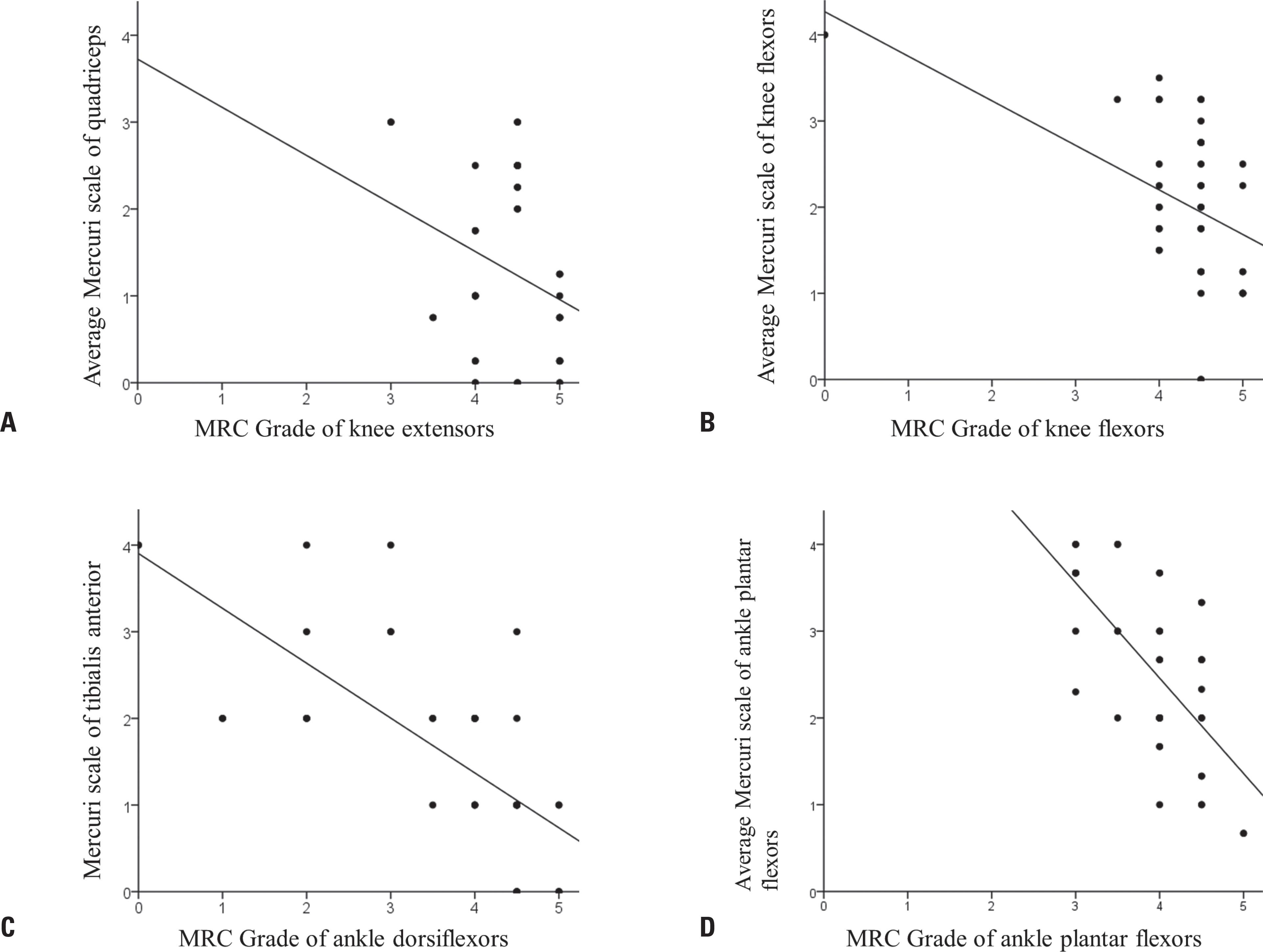
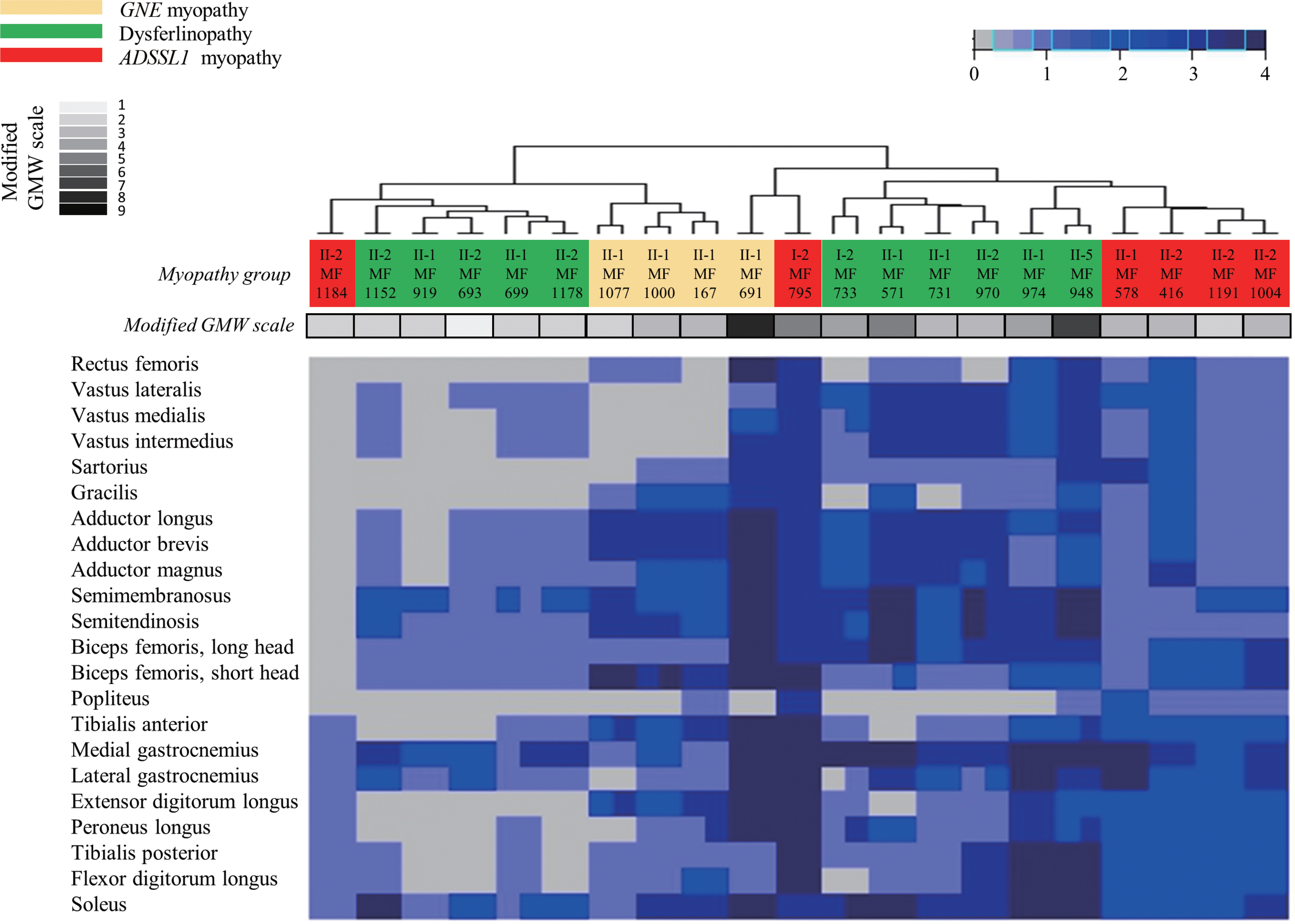
Ten (48%) of the 21 patients were men. The ages of the participants at symptom onset and imaging were 19.2 ± 9.5 and 30.4 ± 9.0 years (mean ± standard deviation), respectively. Their grade on the modified Gardner-Medwin and Walton grade was 3.3 ± 1.7. The strength grade of the knee extensors was not correlated with the Mercuri scale for the quadriceps (r = −0.247, p = 0.115). However, the Medical Research Council grades of the knee flexors, ankle dorsiflexors, and ankle plantar flexors were significantly correlated with the Mercuri scale ratings of the knee flexors (r = −0.497, p = 0.001), tibialis anterior (r = −0.727, p < 0.001), and ankle plantar flexors (r = −0.620, p < 0.001), respectively. T1-weighted MR images showed characteristic fatty replacement patterns that were consistent with the causative genes. Unsupervised hierarchical clustering of the Mercuri scale showed that the main factors contributing to the dichotomy were the causative gene and the clinical severity.
CONCLUSIONS
This study is the first to reveal the usefulness of lower-limb MR images in the differential diagnosis of distal myopathy in Korea.
Keyword
MeSH Terms
Figure
Reference
-
1.Bonne G., Rivier F., Hamroun D. The 2018 version of the gene ta-ble of monogenic neuromuscular disorders (nuclear genome). Neuromuscul Disord. 2017. 27:1152–1183.
Article2.Park HJ., Shin HY., Kim S., Kim SH., Lee Y., Lee JH, et al. Distal myopathy with ADSSL1 mutations in Korean patients. Neuromuscul Disord. 2017. 27:465–472.
Article3.Straub V., Carlier PG., Mercuri E. TREAT-NMD workshop: pattern recognition in genetic muscle diseases using muscle MRI: 25–26 February 2011, Rome, Italy. Neuromuscul Disord. 2012. 22(Suppl 2):S42–S53.4.Díaz-Manera J., Llauger J., Gallardo E., Illa I. Muscle MRI in muscular dystrophies. Acta Myol. 2015. 34:95–108.5.Kinali M., Arechavala-Gomeza V., Cirak S., Glover A., Guglieri M., Feng L, et al. Muscle histology vs MRI in Duchenne muscular dystrophy. Neurology. 2011. 76:346–353.
Article6.Fanin M., Angelini C. Muscle pathology in dysferlin deficiency. Neuropathol Appl Neurobiol. 2002. 28:461–470.
Article7.Fischer D., Clemen CS., Olivé M., Ferrer I., Goudeau B., Roth U, et al. Different early pathogenesis in myotilinopathy compared to primary desminopathy. Neuromuscul Disord. 2006. 16:361–367.
Article8.Jungbluth H., Davis MR., Müller C., Counsell S., Allsop J., Chattopad-hyay A, et al. Magnetic resonance imaging of muscle in congen-ital myopathies associated with RYR1 mutations. Neuromuscul Disord. 2004. 14:785–790.
Article9.Eisenberg I., Avidan N., Potikha T., Hochner H., Chen M., Olender T, et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylman-nosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001. 29:83–87.
Article10.Sim JE., Park HJ., Shin HY., Nam TS., Kim SM., Choi YC. Clinical characteristics and molecular genetic analysis of Korean patients with GNE myopathy. Yonsei Med J. 2013. 54:578–582.
Article11.Liu J., Aoki M., Illa I., Wu C., Fardeau M., Angelini C, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998. 20:31–36.
Article12.Park HJ., Hong JM., Suh GI., Shin HY., Kim SM., Sunwoo IN, et al. Het-erogeneous characteristics of Korean patients with dysferlinopathy. J Korean Med Sci. 2012. 27:423–429.
Article13.Paradas C., Llauger J., Diaz-Manera J., Rojas-García R., De Luna N., Iturriaga C, et al. Redefining dysferlinopathy phenotypes based on clinical findings and muscle imaging studies. Neurology. 2010. 75:316–323.
Article14.Tasca G., Ricci E., Monforte M., Laschena F., Ottaviani P., Rodolico C, et al. Muscle imaging findings in GNE myopathy. J Neurol. 2012. 259:1358–1365.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distal Myopathy of Miyoshi Type: 1 Case
- Autosomal Dominant Centronuclear Myopathy with Unique Clinical Presentations
- Progressive Muscular Dystrophy (Report of 32 cases)
- Isolated Distal Leg Weakness due to a Small Cerebral Infarction Masquerading as a Spinal Lesion
- Serially Sectioned Images of the Whole Body (Seventh Report: Segmentation of Lower Limb's Structures in Detail)