Cancer Res Treat.
2016 Apr;48(2):596-604. 10.4143/crt.2015.029.
Appendiceal Neuroendocrine, Goblet and Signet-Ring Cell Tumors: A Spectrum of Diseases with Different Patterns of Presentation and Outcome
- Affiliations
-
- 1Department of Hematology and Oncology, Winship Cancer Institute, Emory University, Atlanta, GA, USA. wshaib@emory.edu
- 2Division of Hematology Oncology, The Ohio State University, Columbus, OH, USA.
- 3Department of Biostatistics, Emory University, Atlanta, GA, USA.
- 4School of Public Health, Emory University, Atlanta, GA, USA.
- 5Department of Pathology, The Ohio State University, Columbus, OH, USA.
- 6Division of Surgical Oncology, Winship Cancer Institute, Emory University, Atlanta, GA, USA.
- 7Department of Surgical Oncology, The Ohio State University, Columbus, OH, USA.
- KMID: 2454337
- DOI: http://doi.org/10.4143/crt.2015.029
Abstract
- PURPOSE
Appendiceal tumors are a heterogeneous group of diseases that include typical neuroendocrine tumors (TNET), goblet cell carcinoids (GCC), and atypical GCC. Atypical GCC are classified into signet-ring cell cancers (SRCC) and poorly differentiated appendiceal adenocarcinoids. The prognosis and management of these diseases is unclear because there are no prospective studies. The aim of this study is to assess the characteristics and outcome of appendiceal TNET, GCC, and SRCC patients.
MATERIALS AND METHODS
Appendiceal TNET, GCC, and SRCC patients diagnosed between 1973 and 2011 were identified in the Surveillance Epidemiology and End Results (SEER) database. Demographics, type of surgery, and clinicopathologic characteristics were collected. Survival functions were estimated by the Kaplan-Meier method, and log-rank test was used to assess the difference in overall survival (OS) among the three histologies.
RESULTS
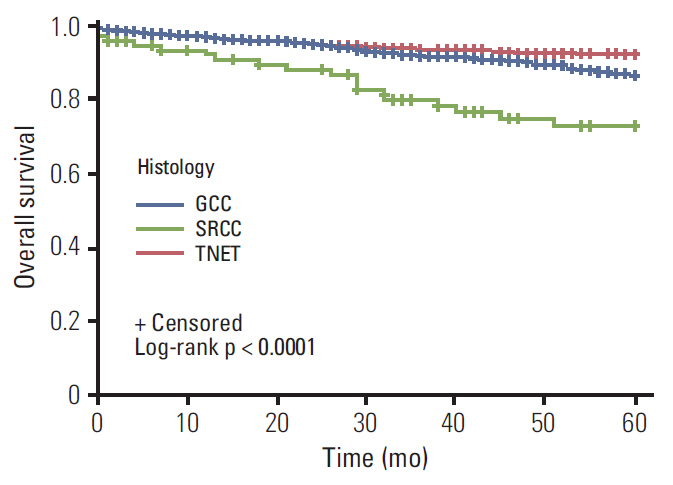
The SEER database yielded 1,021 TNET patients, 1,582 with GCC, and 534 SRCC patients. TNET presented at a younger age (p < 0.001). Patients with SRCC presented with advanced stage disease (p < 0.001). The median OS (mOS) for GCC and TNET patients was not reached; mOS for SRCC was 24 months. Multivariate analysis stratified for stage revealed significantly longer survival for TNET and GCC than SRCC (p < 0.001).
CONCLUSION
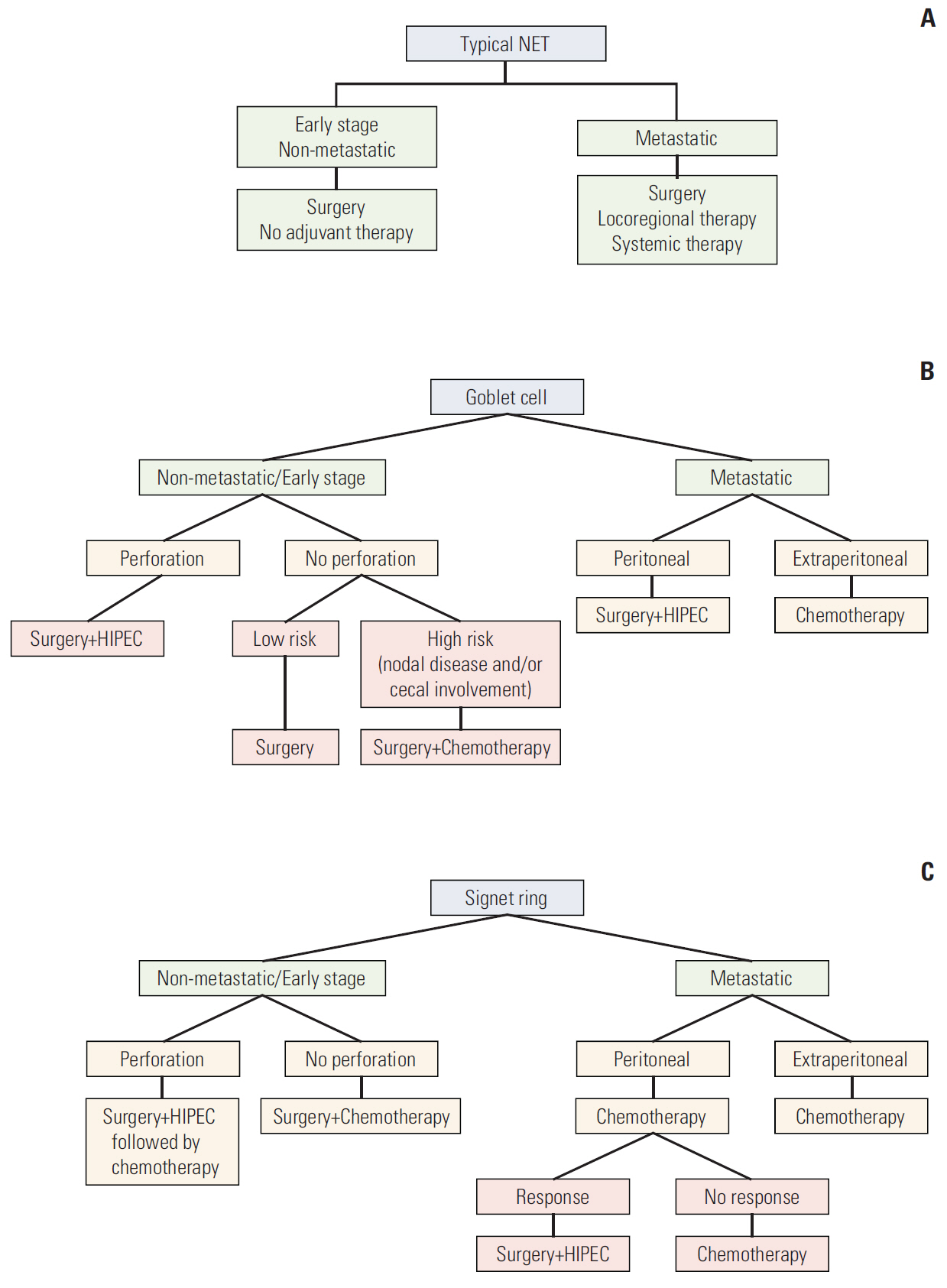
This is the largest report to date for appendiceal neuroendocrine tumor patients, suggesting a spectrum of diseases with different characteristics and outcomes. In this report, we present a treatment approach for this complex spectrum of disease, based on the experience of Ohio State and Emory Universities investigators.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Tang LH, Shia J, Soslow RA, Dhall D, Wong WD, O'Reilly E, et al. Pathologic classification and clinical behavior of the spectrum of goblet cell carcinoid tumors of the appendix. Am J Surg Pathol. 2008; 32:1429–43.
Article2. Burke AP, Sobin LH, Federspiel BH, Shekitka KM, Helwig EB. Goblet cell carcinoids and related tumors of the vermiform appendix. Am J Clin Pathol. 1990; 94:27–35.
Article3. Sugarbaker PH. Cytoreductive surgery and perioperative intraperitoneal chemotherapy: a new standard of care for appendiceal mucinous tumors with peritoneal dissemination. Clin Colon Rectal Surg. 2005; 18:204–14.
Article4. McCusker ME, Cote TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. 2002; 94:3307–12.5. Wang HL, Dhall D. Goblet or signet ring cells: that is the question. Adv Anat Pathol. 2009; 16:247–54.6. National Cancer Institute. SEER as a research resource. Bethesda, MD: National Institutes of Health;2010.7. Abt AB, Carter SL. Goblet cell carcinoid of the appendix: an ultrastructural and histochemical study. Arch Pathol Lab Med. 1976; 100:301–6.8. Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley and Sons;1980.9. Cox DR. Regression models and life-tables. J R Stat Soc Ser B Methodol. 1972; 34:187–220.
Article10. Pahlavan PS, Kanthan R. Goblet cell carcinoid of the appendix. World J Surg Oncol. 2005; 3:36.
Article11. Hsu C, Rashid A, Xing Y, Chiang YJ, Chagpar RB, Fournier KF, et al. Varying malignant potential of appendiceal neuroendocrine tumors: importance of histologic subtype. J Surg Oncol. 2013; 107:136–43.
Article12. Pham TH, Wolff B, Abraham SC, Drelichman E. Surgical and chemotherapy treatment outcomes of goblet cell carcinoid: a tertiary cancer center experience. Ann Surg Oncol. 2006; 13:370–6.
Article13. Shaib WL, Martin LK, Choi M, Chen Z, Krishna K, Kim S, et al. Hyperthermic intraperitoneal chemotherapy following cytoreductive surgery improves outcome in patients with primary appendiceal mucinous adenocarcinoma: a pooled analysis from three tertiary care centers. Oncologist. 2015; 20:907–14.
Article14. Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012; 30:2449–56.
Article15. Moertel CG, Weiland LH, Nagorney DM, Dockerty MB. Carcinoid tumor of the appendix: treatment and prognosis. N Engl J Med. 1987; 317:1699–701.
Article16. Syracuse DC, Perzin KH, Price JB, Wiedel PD, Mesa-Tejada R. Carcinoid tumors of the appendix. Mesoappendiceal extension and nodal metastases. Ann Surg. 1979; 190:58–63.
Article17. Boudreaux JP, Klimstra DS, Hassan MM, Woltering EA, Jensen RT, Goldsmith SJ, et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the jejunum, ileum, appendix, and cecum. Pancreas. 2010; 39:753–66.18. Pape UF, Perren A, Niederle B, Gross D, Gress T, Costa F, et al. ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejunoileum and the appendix including goblet cell carcinomas. Neuroendocrinology. 2012; 95:135–56.
Article19. Park K, Blessing K, Kerr K, Chetty U, Gilmour H. Goblet cell carcinoid of the appendix. Gut. 1990; 31:322–4.
Article20. Edmonds P, Merino MJ, LiVolsi VA, Duray PH. Adenocarcinoid (mucinous carcinoid) of the appendix. Gastroenterology. 1984; 86:302–9.
Article21. Chen V, Qizilbash AH. Goblet cell carcinoid tumor of the appendix: report of five cases and review of the literature. Arch Pathol Lab Med. 1979; 103:180–2.22. Butler JA, Houshiar A, Lin F, Wilson SE. Goblet cell carcinoid of the appendix. Am J Surg. 1994; 168:685–7.
Article23. Goldstein DA, Elvin JA, Wang K, Shaib WL, Rossi MR, Stephens PJ, et al. Comprehensive genomic profiling of cancer of the appendix to reveal new routes to targeted therapies. J Clin Oncol. 2015; 33 Suppl 3:608.
Article24. Stancu M, Wu TT, Wallace C, Houlihan PS, Hamilton SR, Rashid A. Genetic alterations in goblet cell carcinoids of the vermiform appendix and comparison with gastrointestinal carcinoid tumors. Mod Pathol. 2003; 16:1189–98.
Article25. Dimmler A, Geddert H, Faller G. EGFR, KRAS, BRAF-mutations and microsatellite instability are absent in goblet cell carcinoids of the appendix. Pathol Res Pract. 2014; 210:274–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary multifocal cystic signet ring neuroendocrine tumor of liver: a case report
- Primary Signet Ring Cell Adenocarcinoma of the Prostate
- A rare goblet cell adenocarcinoma arising from Barrett’s esophagus: the first reported case in the esophagus
- Cytologic Heterogeneity of Signet Ring Cell Carcinoma of the Stomach: Histochemical and electron microscopic observations
- Cytologic Features of Signet Ring Cell Carcinoma of the Uterine Cervix: A Report of Two Cases