Cancer Res Treat.
2016 Apr;48(2):458-464. 10.4143/crt.2015.135.
Tolerability and Outcomes of First-Line Pemetrexed-Cisplatin Followed by Gefitinib Maintenance Therapy Versus Gefitinib Monotherapy in Korean Patients with Advanced Nonsquamous Non-small Cell Lung Cancer: A Post Hoc Descriptive Subgroup Analysis of a Randomized, Phase 3 Trial
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Seoul St. Mary's Hospital, Seoul, Korea.
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Seoul, Korea. keunchil.park@samsung.com
- 3Division of Hematology-Oncology, Department of Medicine, Seoul National University Hospital, Seoul, Korea.
- 4Division of Hematology-Oncology, Department of Medicine, Gachon University Gil Hospital, Incheon, Korea.
- 5Division of Hematology-Oncology, Department of Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 6Division of Hematology-Oncology, Department of Medicine, Korea University Anam Hospital, Seoul, Korea.
- 7Eli Lilly and Company, Shanghai, China.
- 8Eli Lilly and Company, Seoul, Korea.
- 9Eli Lilly Interamerica Inc., Buenos Aires, Argentina.
- KMID: 2454321
- DOI: http://doi.org/10.4143/crt.2015.135
Abstract
- PURPOSE
We recently reported on a randomized, open-label, phase 3 trial comparing pemetrexed-cisplatin chemotherapy followed by gefitinib maintenance therapy (PC/G) with gefitinib monotherapy in patients with non-small cell lung cancer (NSCLC). Here, we report on a post hoc subgroup analysis of that study assessing the demographics and disposition of the Korean patient subgroup, and comparing the tolerability of PC/G and gefitinib monotherapy and the tumor response with respect to epidermal growth factor receptor (EGFR) status.
MATERIALS AND METHODS
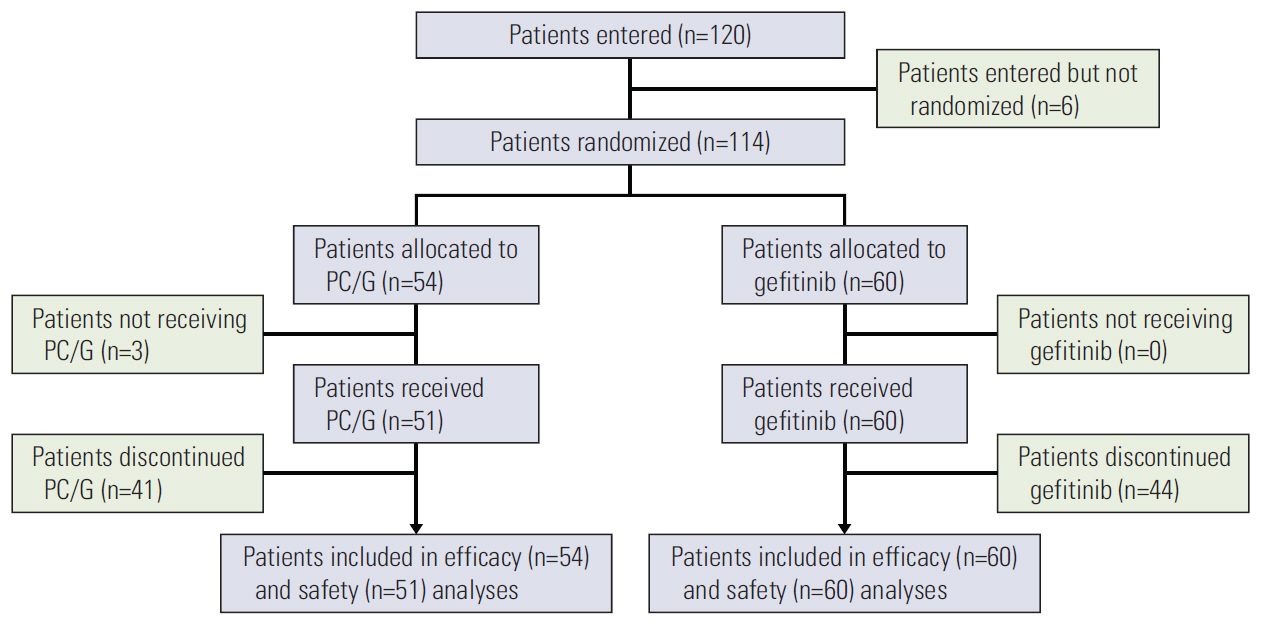
Patients, who were ≥ 18 years, chemonaïve, Korean, light ex-smokers/never-smokers with advanced NSCLC, were randomly assigned (1:1) to PC/G or gefitinib monotherapy. Treatment-emergent adverse events (TEAEs) were graded, and tumor response was measured as change in lesion sum from baseline at best response. The study was registered with ClinicalTrials. gov, NCT01017874.
RESULTS
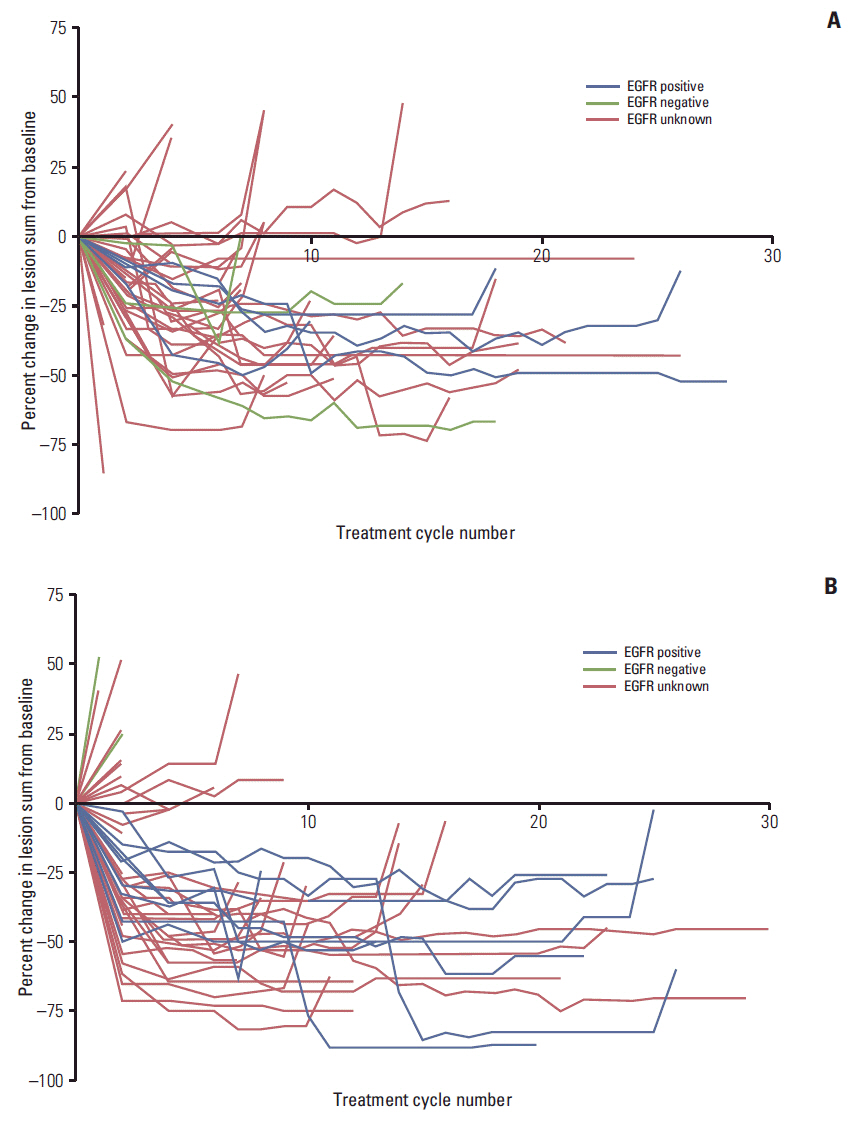
Overall, 111 Korean patients were treated (PC/G, 51; gefitinib, 60). Between-arm characteristics were balanced and similar to those of the overall population. Treatment discontinuations due to adverse events were low (PC/G: 1, 2.0%; gefitinib: 7, 11.7%). Overall, 92 patients (82.9%) reported ≥ 1 TEAE (PC/G, 44; gefitinib, 48); few patients (PC/G, 16; gefitinib, 7) reported severe TEAEs; the most frequent was neutropenia (PC/G arm) and elevated alanine aminotransferase (gefitinib arm). The lesion sum was decreased by PC/G treatment in most patients, regardless of EGFR mutation status, while gefitinib monotherapy reduced the lesion sum in EGFR-positive patients but had no effect in EGFR-negative patients.
CONCLUSION
Our results confirm that both PC/G and gefitinib were well tolerated in Korean patients, regardless of EGFR status; however, patients with EGFR wild-type NSCLC may not benefit from gefitinib monotherapy.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ferlay J, Soerjomataram I, Ervik M, Forman D, Bray F, Dikshit R, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon: International Agency for Research on Cancer;2013 [cited 2015 Mar 24]. Available from: http://globocan.iarc.fr.2. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Prediction of cancer incidence and mortality in Korea, 2014. Cancer Res Treat. 2014; 46:124–30.
Article3. D'Addario G, Fruh M, Reck M, Baumann P, Klepetko W, Felip E, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21 Suppl 5:v116–9.4. Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, Brahmer J, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009; 27:6251–66.
Article5. Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013; 137:828–60.
Article6. Socinski MA, Evans T, Gettinger S, Hensing TA, Sequist LV, Ireland B, et al. Treatment of stage IV non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; 143(5 Suppl):e341S–68S.7. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.
Article8. Ahn MJ, Yang JC, Liang J, Kang JH, Xiu Q, Chen YM, et al. Randomized phase II trial of first-line treatment with pemetrexedcisplatin, followed sequentially by gefitinib or pemetrexed, in East Asian, never-smoker patients with advanced non-small cell lung cancer. Lung Cancer. 2012; 77:346–52.
Article9. Yang JC, Kang JH, Mok T, Ahn MJ, Srimuninnimit V, Lin CC, et al. First-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian patients with locally advanced or metastatic non-squamous non-small cell lung cancer: a randomised, phase 3 trial. Eur J Cancer. 2014; 50:2219–30.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pemetrexed Continuation Maintenance in Patients with Nonsquamous Non-small Cell Lung Cancer: Review of Two East Asian Trials in Reference to PARAMOUNT
- Randomized Phase II Study of Pemetrexed Versus Gefitinib in Previously Treated Patients with Advanced Non-small Cell Lung Cancer
- Comparison of Gefitinib versus Docetaxel in Patients with Pre-Treated Non-Small Cell Lung Cancer (NSCLC)
- Post-Progression Survival in Patients with Non-Small Cell Lung Cancer with Clinically Acquired Resistance to Gefitinib
- Pemetrexed-Erlotinib, Pemetrexed Alone, or Erlotinib Alone as Second-Line Treatment for East Asian and Non-East Asian Never-Smokers with Locally Advanced or Metastatic Nonsquamous Non-small Cell Lung Cancer: Exploratory Subgroup Analysis of a Phase II Trial