Yonsei Med J.
2012 Sep;53(5):931-939.
Prognostic and Predictive Value of Carcinoembryonic Antigen and Cytokeratin-19 Fragments Levels in Advanced Non-Small Cell Lung Cancer Patients Treated with Gefitinib or Erlotinib
- Affiliations
-
- 1Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. cbc1971@yuhs.ac
- 2Division of Medical Oncology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Division of Pulmonology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Thoracic and Cardiovascular Surgery, Yonsei University College of Medicine, Seoul, Korea. kdjcool@yuhs.ac
Abstract
- PURPOSE
The prognostic and predictive value of pretreatment serum levels of carcinoembryonic antigen (CEA) and cytokeratin-19 fragments (CYFRA 21-1) were assessed in advanced non-small cell lung cancer (NSCLC) patients treated with gefitinib or erlotinib.
MATERIALS AND METHODS
Pretreatment CEA and CYFRA 21-1 were measured in 123 advanced NSCLC patients receiving gefitinib or erlotinib. High CEA levels (h-CEA) were significantly associated with females, patients with adenocarcinoma, and non-smokers.
RESULTS
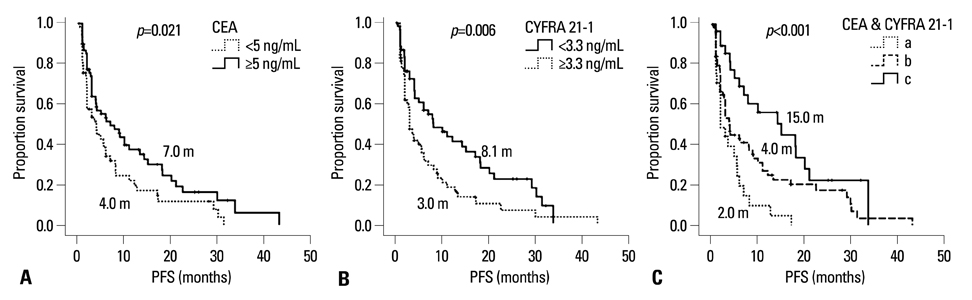
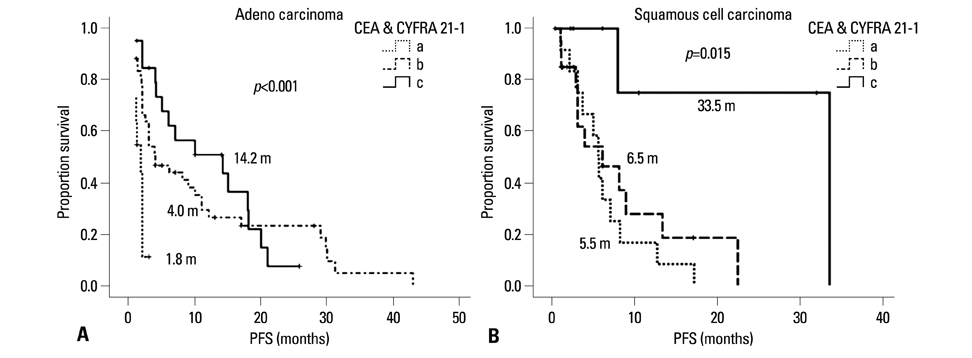
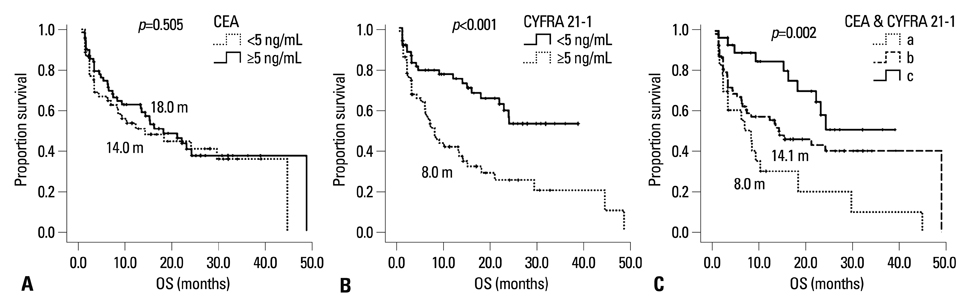
Low CYFRA 21-1 levels (l-CYFRA) were significantly associated with a good performance status (ECOG PS 0-1). The overall response rate (RR) was 27.6%, and higher RR was associated with adenocarcinoma, h-CEA, and epidermal growth factor receptor (EGFR) mutation. Patients with h-CEA had significantly longer progression-free survival (PFS) (p=0.021). Patients with l-CYFRA had significantly longer PFS and overall survival (p=0.006 and p<0.001, respectively). Of note, h-CEA and l-CYFRA had good prognosis in patients with unknown EGFR mutation status or patients with squamous cell carcinoma (p=0.021 and p=0.015, respectively). A good ECOG PS (HR=0.45, p=0.017), h-CEA (HR=0.41, p=0.007), l-CYFRA 21-1 (HR=0.52, p=0.025), and an EGFR mutation (HR=0.22, p<0.001) were independently predictive of a longer PFS.
CONCLUSION
h-CEA and l-CYFRA 21-1 may be prognostic and predictive serum markers for higher response and longer survival in patients with advanced NSCLC receiving gefitinib or erlotinib, especially in patients with unknown EGFR mutation status or patients with squamous cell carcinoma.
Keyword
MeSH Terms
-
Adenocarcinoma
Biomarkers
Carcinoembryonic Antigen*
Carcinoma, Non-Small-Cell Lung*
Carcinoma, Squamous Cell
Disease-Free Survival
Erlotinib Hydrochloride*
Female
Humans
Keratin-19*
Prognosis
Receptor, Epidermal Growth Factor
Biomarkers
Carcinoembryonic Antigen
Erlotinib Hydrochloride
Keratin-19
Receptor, Epidermal Growth Factor
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008. 58:71–96.
Article2. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005. 353:123–132.
Article3. Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008. 372:1809–1818.
Article4. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009. 361:947–957.
Article5. Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008. 359:366–377.
Article6. Molina R, Auge JM, Escudero JM, Marrades R, Viñolas N, Carcereny E, et al. Mucins CA 125, CA 19.9, CA 15.3 and TAG-72.3 as tumor markers in patients with lung cancer: comparison with CYFRA 21-1, CEA, SCC and NSE. Tumour Biol. 2008. 29:371–380.
Article7. Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, et al. Prognostic significance of perioperative serum carcinoembryonic antigen in non-small cell lung cancer: analysis of 1,000 consecutive resections for clinical stage I disease. Ann Thorac Surg. 2004. 78:216–221.
Article8. Pujol JL, Boher JM, Grenier J, Quantin X. Cyfra 21-1, neuron specific enolase and prognosis of non-small cell lung cancer: prospective study in 621 patients. Lung Cancer. 2001. 31:221–231.
Article9. Holdenrieder S, von Pawel J, Dankelmann E, Duell T, Faderl B, Markus A, et al. Nucleosomes and CYFRA 21-1 indicate tumor response after one cycle of chemotherapy in recurrent non-small cell lung cancer. Lung Cancer. 2009. 63:128–135.
Article10. Ardizzoni A, Cafferata MA, Tiseo M, Filiberti R, Marroni P, Grossi F, et al. Decline in serum carcinoembryonic antigen and cytokeratin 19 fragment during chemotherapy predicts objective response and survival in patients with advanced nonsmall cell lung cancer. Cancer. 2006. 107:2842–2849.
Article11. Okamoto T, Nakamura T, Ikeda J, Maruyama R, Shoji F, Miyake T, et al. Serum carcinoembryonic antigen as a predictive marker for sensitivity to gefitinib in advanced non-small cell lung cancer. Eur J Cancer. 2005. 41:1286–1290.
Article12. Barlési F, Tchouhadjian C, Doddoli C, Torre JP, Astoul P, Kleisbauer JP. CYFRA 21-1 level predicts survival in non-small-cell lung cancer patients receiving gefitinib as third-line therapy. Br J Cancer. 2005. 92:13–14.
Article13. Chiu CH, Shih YN, Tsai CM, Liou JL, Chen YM, Perng RP. Serum tumor markers as predictors for survival in advanced non-small cell lung cancer patients treated with gefitinib. Lung Cancer. 2007. 57:213–221.
Article14. Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. Histological typing of lung and pleural tumours. 1999. 3rd ed. Berlin: Springer-Verlag.15. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.16. Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005. 23:2493–2501.
Article17. Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965. 121:439–462.
Article18. Fujishima T, Honda Y, Shijubo N, Takahashi H, Abe S. Increased carcinoembryonic antigen concentrations in sera and bronchoalveolar lavage fluids of patients with pulmonary alveolar proteinosis. Respiration. 1995. 62:317–321.
Article19. Rule AH, Straus E, Vandevoorde J, Janowitz HD. Tumor-associated (CEA-reacting) antigen in patients with inflammatory bowel disease. N Engl J Med. 1972. 287:24–26.
Article20. Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, et al. Effect of histologic type and smoking status on interpretation of serum carcinoembryonic antigen value in non-small cell lung carcinoma. Ann Thorac Surg. 2004. 78:1004–1009.
Article21. Matsuoka K, Sumitomo S, Nakashima N, Nakajima D, Misaki N. Prognostic value of carcinoembryonic antigen and CYFRA21-1 in patients with pathological stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2007. 32:435–439.
Article22. Shoji F, Yoshino I, Yano T, Kometani T, Ohba T, Kouso H, et al. Serum carcinoembryonic antigen level is associated with epidermal growth factor receptor mutations in recurrent lung adenocarcinomas. Cancer. 2007. 110:2793–2798.
Article23. Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989. 57:327–334.
Article24. Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999. 9:67–81.
Article25. Screaton RA, Penn LZ, Stanners CP. Carcinoembryonic antigen, a human tumor marker, cooperates with Myc and Bcl-2 in cellular transformation. J Cell Biol. 1997. 137:939–952.
Article26. Ordoñez C, Screaton RA, Ilantzis C, Stanners CP. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 2000. 60:3419–3424.27. Stieber P, Bodenmüller H, Banauch D, Hasholzner U, Dessauer A, Ofenloch-Hähnle B, et al. Cytokeratin 19 fragments: a new marker for non-small-cell lung cancer. Clin Biochem. 1993. 26:301–304.
Article28. Nisman B, Lafair J, Heching N, Lyass O, Baras M, Peretz T, et al. Evaluation of tissue polypeptide specific antigen, CYFRA 21-1, and carcinoembryonic antigen in nonsmall cell lung carcinoma: does the combined use of cytokeratin markers give any additional information? Cancer. 1998. 82:1850–1859.
Article29. Barlési F, Gimenez C, Torre JP, Doddoli C, Mancini J, Greillier L, et al. Prognostic value of combination of Cyfra 21-1, CEA and NSE in patients with advanced non-small cell lung cancer. Respir Med. 2004. 98:357–362.
Article30. Pujol JL, Molinier O, Ebert W, Daurès JP, Barlesi F, Buccheri G, et al. CYFRA 21-1 is a prognostic determinant in non-small-cell lung cancer: results of a meta-analysis in 2063 patients. Br J Cancer. 2004. 90:2097–2105.
Article31. Kao CH, Hsieh JF, Ho YJ, Tsai SC, Lee JK. Cytokeratin fragment 19 (CYFRA 21-1) in healthy smokers. Anticancer Res. 1999. 19:4545–4546.32. Molina R, Filella X, Augé JM, Fuentes R, Bover I, Rifa J, et al. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol. 2003. 24:209–218.
Article33. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005. 97:339–346.
Article34. Gaughan EM, Costa DB. Genotype-driven therapies for non-small cell lung cancer: focus on EGFR, KRAS and ALK gene abnormalities. Ther Adv Med Oncol. 2011. 3:113–125.
Article35. Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011. 12:1004–1012.
Article36. Herbst RS, Blumenschein GR Jr, Kim ES, Lee J, Tsao AS, Alden CM, et al. Sorafenib treatment efficacy and KRAS biomarker status in the Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE) trial. J Clin Oncol. 2010. 28:15 Suppl. 7609.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retraction: Prognostic and Predictive Value of Carcinoembryonic Antigen and Cytokeratin-19 Fragments Levels in Advanced Non-Small Cell Lung Cancer Patients Treated with Gefitinib or Erlotinib. Yonsei Med J 2012;53:931-9.
- Efficacy and Safety of Afatinib for EGFR-mutant Non-small Cell Lung Cancer, Compared with Gefitinib or Erlotinib
- Comparison of the therapeutic outcome between gefitinib and erlotinib in female patients with non-small-cell lung cancer
- Serum Carcinoembryonic Antigen as an Index of the Therapeutic Effect of EGFR-TKIs in Patients with Advanced Non-Small Cell Lung Cancer
- Clinical efficacy of erlotinib, a salvage treatment for non-small cell lung cancer patients following gefitinib failure