Korean J Transplant.
2019 Jun;33(2):20-29. 10.4285/jkstn.2019.33.2.20.
Efficacy and safety of prolonged-release versus immediate-release tacrolimus in de novo liver transplant recipients in South Korea: a randomized open-label phase 4 study (MAPLE)
- Affiliations
-
- 1Department of Transplant Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. soonkim@yuhs.ac
- 2Department of Surgery, Samsung Medical Center, Seoul, Korea.
- 3Department of Surgery, Korea University College of Medicine, Seoul, Korea.
- 4Department of Hepatobiliary Surgery, National Cancer Center, Seoul, Korea.
- 5Department of Hepatobiliary-Pancreas Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Surgery, Kangbuk Samsung Hospital, Seoul, Korea.
- 7Department of Hepatobiliary Surgery, Korea University Anam Hospital, Seoul, Korea.
- 8Department of Hepatobiliary Surgery, Asan Medical Center, Seoul, Korea.
- 9Department of Surgery, St. Vincent's Hospital, The Catholic University of Korea, Seoul, Korea.
- 10Astellas Pharma Inc., Seoul, Korea.
- 11Astellas Pharma Inc., Singapore.
- KMID: 2453795
- DOI: http://doi.org/10.4285/jkstn.2019.33.2.20
Abstract
- BACKGROUND
Prolonged-release tacrolimus is associated with better long-term graft and patient survival than the immediate-release formulation in liver transplant patients. However, no clinical data are available to assess the efficacy and safety of early conversion from twice-daily, immediate-release tacrolimus to once-daily, prolonged-release tacrolimus in de novo liver transplant recipients in Korea.
METHODS
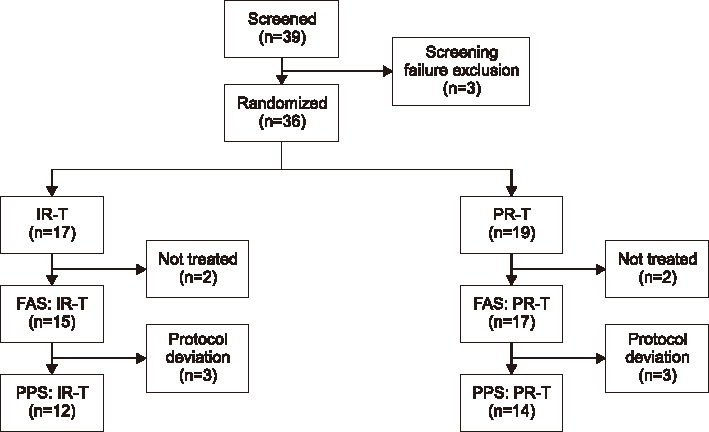
A 24-week, randomized, open-label study was conducted in 36 liver transplant recipients. All patients received immediate-release tacrolimus (0.1-0.2 mg/kg/day, divided into two doses) for 4 weeks after transplantation, at which time 50% of the patients were converted, at a ratio of 1 mg to 1 mg, to prolonged-release tacrolimus (once-daily). The primary efficacy endpoint was the incidence of biopsy-confirmed acute rejection (BCAR) from weeks 4 to 24 after transplantation (per-protocol set). Medication adherence, adverse event profiles, laboratory tests, vital signs, and physical changes were also recorded.
RESULTS
BCAR frequency at 24 weeks was similar between the two treatment groups; two cases (mean±standard deviation, 0.14±0.53 cases) of BCAR were reported in one patient treated with prolonged-release tacrolimus (n=14), while no such cases were reported among patients treated with immediate-release tacrolimus (n=12). The tacrolimus blood concentration at weeks 12 and 24, medication adherence, and adverse event profiles were also similar between the formulations, with no unusual laboratory test results, vital signs, or physical changes reported.
CONCLUSIONS
Early conversion to a simplified, once-daily, prolonged-release tacrolimus regimen may be an effective treatment option for liver transplant recipients in Korea. Larger-scale studies are warranted to confirm non-inferiority to immediate-release tacrolimus formulation in de novo liver transplant recipients.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Harper AM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant. 2018; 18:Suppl 1. 172–253.
Article2. Adam R, Karam V, Delvart V, Trunečka P, Samuel D, Bechstein WO, et al. Improved survival in liver transplant recipients receiving prolonged-release tacrolimus in the European Liver Transplant Registry. Am J Transplant. 2015; 15:1267–1282.
Article3. Christina S, Annunziato RA, Schiano TD, Anand R, Vaidya S, Chuang K, et al. Medication level variability index predicts rejection, possibly due to nonadherence, in adult liver transplant recipients. Liver Transpl. 2014; 20:1168–1177.
Article4. Beckebaum S, Iacob S, Sweid D, Sotiropoulos GC, Saner F, Kaiser G, et al. Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transpl Int. 2011; 24:666–675.
Article5. Rodrigue JR, Nelson DR, Hanto DW, Reed AI, Curry MP. Patient-reported immunosuppression nonadherence 6 to 24 months after liver transplant: association with pretransplant psychosocial factors and perceptions of health status change. Prog Transplant. 2013; 23:319–328.
Article6. Trunečka P, Boillot O, Seehofer D, Pinna AD, Fischer L, Ericzon BG, et al. Once-daily prolonged-release tacrolimus (ADVAGRAF) versus twice-daily tacrolimus (PROGRAF) in liver transplantation. Am J Transplant. 2010; 10:2313–2323.
Article7. Considine A, Tredger JM, Heneghan M, Agarwal K, Samyn M, Heaton ND, et al. Performance of modified-release tacrolimus after conversion in liver transplant patients indicates potentially favorable outcomes in selected cohorts. Liver Transpl. 2015; 21:29–37.
Article8. Stifft F, Stolk LM, Undre N, van Hooff JP, Christiaans MH. Lower variability in 24-hour exposure during once-daily compared to twice-daily tacrolimus formulation in kidney transplantation. Transplantation. 2014; 97:775–780.
Article9. Wu MJ, Chang CH, Cheng CY, Shu KH, Chen CH, Cheng CH, et al. Reduced variability of tacrolimus trough level in once-daily tacrolimus-based Taiwanese kidney transplant recipients with high-expressive genotype of cytochrome P450 3A5. Transplant Proc. 2014; 46:403–405.
Article10. Kuypers DR, Peeters PC, Sennesael JJ, Kianda MN, Vrijens B, Kristanto P, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013; 95:333–340.
Article11. Eberlin M, Otto G, Krämer I. Increased medication compliance of liver transplant patients switched from a twice-daily to a once-daily tacrolimus-based immunosuppressive regimen. Transplant Proc. 2013; 45:2314–2320.
Article12. Dopazo C, Rodriguez R, Llado L, Calatayud D, Castells L, Ramos E, et al. Successful conversion from twice-daily to once-daily tacrolimus in liver transplantation: observational multicenter study. Clin Transplant. 2012; 26:E32–E37.
Article13. Dumortier J, Guillaud O, Boillot O. Conversion from twice daily tacrolimus to once daily tacrolimus in long-term stable liver transplant recipients: a single-center experience with 394 patients. Liver Transpl. 2013; 19:529–533.
Article14. Sańko-Resmer J, Boillot O, Wolf P, Thorburn D. Renal function, efficacy and safety postconversion from twice- to once-daily tacrolimus in stable liver recipients: an open-label multicenter study. Transpl Int. 2012; 25:283–293.
Article15. Valente G, Rinaldi L, Sgambato M, Piai G. Conversion from twice-daily to once-daily tacrolimus in stable liver transplant patients: effectiveness in a real-world setting. Transplant Proc. 2013; 45:1273–1275.
Article16. Kim JM, Kwon CH, Joh JW, Sinn DH, Lee S, Choi GS, et al. Conversion of once-daily extended-release tacrolimus is safe in stable liver transplant recipients: a randomized prospective study. Liver Transpl. 2016; 22:209–216.
Article17. Kim SH, Lee SD, Kim YK, Park SJ. Conversion of twice-daily to once-daily tacrolimus is safe in stable adult living donor liver transplant recipients. Hepatobiliary Pancreat Dis Int. 2015; 14:374–379.
Article18. Astellas Pharma Europe. Advagraf 0.5 mg, 1 mg, 3 mg and 5 mg prolonged-release hard capsules summary of product characteristics. Leiden, NL: Astellas Pharma;2015.19. U.S. Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994; 331:1110–1115.20. Margarit C, Bilbao I, Castells L, Lopez I, Pou L, Allende E, et al. A prospective randomized trial comparing tacrolimus and steroids with tacrolimus monotherapy in liver transplantation: the impact on recurrence of hepatitis C. Transpl Int. 2005; 18:1336–1345.
Article21. Jain AB, Kashyap R, Rakela J, Starzl TE, Fung JJ. Primary adult liver transplantation under tacrolimus: more than 90 months actual follow-up survival and adverse events. Liver Transpl Surg. 1999; 5:144–150.
Article22. TruneČka P, Klempnauer J, Bechstein WO, Pirenne J, Friman S, Zhao A, et al. Renal function in de novo liver transplant recipients receiving different prolonged-release tacrolimus regimens: the DIAMOND Study. Am J Transplant. 2015; 15:1843–1854.
Article23. Uemoto S, Abe R, Horike H, So M. Safety and efficacy of once-daily modified-release tacrolimus in liver transplant recipients: a multicenter postmarketing surveillance in Japan. Transplant Proc. 2014; 46:749–753.
Article24. Gastaca M, Valdivieso A, Bustamante J, Fernández JR, Ruiz P, Ventoso A, et al. Favorable longterm outcomes of liver transplant recipients treated de novo with once-daily tacrolimus: results of a single-center cohort. Liver Transpl. 2016; 22:1391–1400.
Article25. van Hooff JP, Alloway RR, Trunečka P, Mourad M. Four-year experience with tacrolimus once-daily prolonged release in patients from phase II conversion and de novo kidney, liver, and heart studies. Clin Transplant. 2011; 25:E1–E12.
Article26. Guirado L, Cantarell C, Franco A, Huertas EG, Fructuoso AS, Fernández A, et al. Efficacy and safety of conversion from twice-daily to once-daily tacrolimus in a large cohort of stable kidney transplant recipients. Am J Transplant. 2011; 11:1965–1971.
Article27. Okumura Y, Noda T, Eguchi H, Iwagami Y, Yamada D, Asaoka T, et al. Short- and long-term outcomes of de novo liver transplant patients treated with once-daily prolonged-release tacrolimus. Transplant Direct. 2017; 3:e207.
Article28. Coilly A, Calmus Y, Chermak F, Dumortier J, Duvoux C, Guillaud O, et al. Once-daily prolonged release tacrolimus in liver transplantation: experts' literature review and recommendations. Liver Transpl. 2015; 21:1312–1321.
Article29. Han N, Ha S, Yun HY, Kim MG, Min SI, Ha J, et al. Population pharmacokinetic-pharmacogenetic model of tacrolimus in the early period after kidney transplantation. Basic Clin Pharmacol Toxicol. 2014; 114:400–406.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multicenter Clinical Investigation for the Safety and Efficacy of Advagraf(R) (Extended Release Tacrolimus) versus Prograf(R) (Tacrolimus) in De Novo Kidney Recipients after 1 Month of Transplantation: Preliminary Results
- Efficacy and safety of generic once-daily prolonged release tacrolimus (TacroBell SR cap.) in de novo kidney transplant recipients: a multicenter, non-comparative, phase IV study
- A noninferiority, randomized controlled trial of late conversion to once-daily regimen of sirolimus and extended-release tacrolimus versus mycophenolic acid and extended-release tacrolimus for kidney transplant recipients
- Risk of graft loss on once-daily versus twice-daily tacrolimus in kidney transplant patients: a meta-analysis
- Evaluation of the efficacy and safety of conversion from the tacrolimus capsule to tablet in stable liver transplant recipients with maintenance therapy: a 24-week, open-label, single-center, phase IV exploratory clinical study