Electrolyte Blood Press.
2019 Jun;17(1):1-6. 10.5049/EBP.2019.17.1.1.
Pharmacologic Treatment of Chronic Hyperkalemia in Patients with Chronic Kidney Disease
- Affiliations
-
- 1Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea. kimgh@hanyang.ac.kr
- KMID: 2451515
- DOI: http://doi.org/10.5049/EBP.2019.17.1.1
Abstract
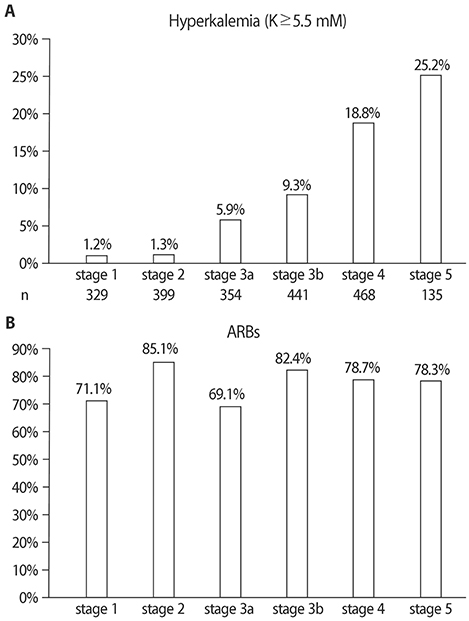
- Hyperkalemia is frequently complicated in patients with advanced chronic kidney disease (CKD) because kidney is the major route of potassium excretion. Urinary potassium excretion is reduced according to the decline in glomerular filtration rate, and the risk of hyperkalemia is increased in patients with high potassium intake, advanced age, diabetes mellitus, congestive heart failure, and medications such as renin-angiotensin-aldosterone system(RAAS) blockades. On the other hand, the benefits of RAAS blockades and a high-potassium diet should be considered in CKD patients. To overcome these contradictory treatment strategies, potassium binders have emerged as new options to enhance fecal potassium excretion. In different regions of the world, four types of potassium binders are preferentially used. Whereas sodium polystyrene sulfonate (SPS) exchanges sodium for potassium, calcium polystyrene sulfonate (CPS) has the advantage of avoiding hypervolemia because it exchanges calcium for potassium. SPS was first introduced in the 1950s and used for a long time in western countries, and CPS is currently prescribed in Asia including South Korea. In contrast with the paucity of clinical studies using SPS or CPS, the recent randomized, controlled trials reported that two newer potassium binders, patiromer and sodium zirconium cyclosilicate (ZS-9), effectively and safely reduce serum potassium levels in CKD patients taking RAAS blockades. Our experiences showed that the long-term administration of a small dose of CPS was also effective and safe in treatment of chronic hyperkalemia. Further comparative trials among patiromer, ZS-9, and CPS are required to provide guides to cost-effective management of hyperkalemia in CKD patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Zacchia M, Abategiovanni ML, Stratigis S, Capasso G. Potassium: from physiology to clinical implications. Kidney Dis (Basel). 2016; 2:72–79.
Article2. Ueda Y, Ookawara S, Ito K, Miyazawa H, Kaku Y, Hoshino T, Tabei K, Morishita Y. Changes in urinary potassium excretion in patients with chronic kidney disease. Kidney Res Clin Pract. 2016; 35:78–83.
Article3. Preston RA, Afshartous D, Garg D, Medrano S, Alonso AB, Rodriguez R. Mechanisms of impaired potassium handling with dual renin-angiotensin-aldosterone blockade in chronic kidney disease. Hypertension. 2009; 53:754–760.
Article4. Bricker NS, Fine LG, Kaplan M, Epstein M, Bourgoignie JJ, Light A. Magnification phenomenon in chronic renal disease. N Engl J Med. 1978; 299:1287–1293.
Article5. Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004; 351:585–592.
Article6. Georgianos PI, Agarwal R. Revisiting RAAS blockade in CKD with newer potassium-binding drugs. Kidney Int. 2018; 93:325–334.
Article7. Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, Hasegawa T, Heerspink HL, Hirayama A, Landman GWD, Levin A, Nitsch D, Wheeler DC, Coresh J, Hallan SI, Shalev V, Grams ME. CKD Prognosis Consortium. Serum potassium and adverse outcomes across the range of kidney function: a CKD prognosis consortium meta-analysis. Eur Heart J. 2018; 39:1535–1542.
Article8. Chauveau P, Koppe L, Combe C, Lasseur C, Trolonge S, Aparicio M. Vegetarian diets and chronic kidney disease. Nephrol Dial Transplant. 2019; 34:199–207.
Article9. Alvo M, Warnock DG. Hyperkalemia. West J Med. 1984; 141:666–671.10. Palmer BF, Clegg DJ. Diagnosis and treatment of hyperkalemia. Cleve Clin J Med. 2017; 84:934–942.
Article11. De Nicola L, Di Lullo L, Paoletti E, Cupisti A, Bianchi S. Chronic hyperkalemia in non-dialysis CKD: controversial issues in nephrology practice. J Nephrol. 2018; 31:653–664.
Article12. Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014; 10:653–662.
Article13. Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016; 89:546–554.
Article14. Sorensen MV, Matos JE, Praetorius HA, Leipziger J. Colonic potassium handling. Pflugers Arch. 2010; 459:645–656.
Article15. Gruy-Kapral C, Emmett M, Santa Ana CA, Porter JL, Fordtran JS, Fine KD. Effect of single dose resin-cathartic therapy on serum potassium concentration in patients with end-stage renal disease. J Am Soc Nephrol. 1998; 9:1924–1930.
Article16. Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013; 126:264.e9–264.e24.
Article17. Lepage L, Dufour AC, Doiron J, Handfield K, Desforges K, Bell R, Vallée M, Savoie M, Perreault S, Laurin LP, Pichette V, Lafrance JP. Randomized clinical ttrial of sodium polystyrene sulfonate for the treatment of mild hyperkalemiain CKD. Clin J Am Soc Nephrol. 2015; 10:2136–2142.
Article18. Beccari MV, Meaney CJ. Clinical utility of patiromer, sodium zirconium cyclosilicate, and sodium polystyrene sulfonate for the treatment of hyperkalemia: an evidence-based review. Core Evid. 2017; 12:11–24.
Article19. Abuelo JG. Treatment of Severe Hyperkalemia: Confronting 4 fallacies. Kidney Int Rep. 2017; 3:47–55.
Article20. Nakayama Y, Ueda K, Yamagishi SI, Sugiyama M, Yoshida C, Kurokawa Y, Nakamura N, Moriyama T, Kodama G, Minezaki T, Ito S, Nagata A, Taguchi K, Yano J, Kaida Y, Shibatomi K, Fukami K. Compared effects of calcium and sodium polystyrene sulfonate on mineral and bone metabolism and volume overload in pre-dialysis patients with hyperkalemia. Clin Exp Nephrol. 2018; 22:35–44.
Article21. Yu MY, Yeo JH, Park JS, Lee CH, Kim GH. Long-term efficacy of oral calcium polystyrene sulfonate for hyperkalemiain CKD patients. PLoS One. 2017; 12(3):e0173542.22. Joo M, Bae WK, Kim NH, Han SR. Colonic mucosal necrosis following administration of calcium polystryrene sulfonate (Kalimate) in a uremic patient. J Korean Med Sci. 2009; 24:1207–1211.
Article23. Huang I. RLY5016: A novel, non-absorbed, therapeutic polymer for serum potassium control. J Am Soc Nephrol. 2010; 21:482A–483A.24. Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One. 2014; 9:e114686.25. Spinowitz BS, Fishbane S, Pergola PE, Roger SD, Lerma EV, Butler J, von Haehling S, Adler SH, Zhao J, Singh B, Lavin PT, McCullough PA, Kosiborod M, Packham DK. ZS-005 Study Investigators. Sodium zirconium cyclosilicateamong individuals with hyperkalemia: A 12-month phase 3 study. Clin J Am Soc Nephrol. 2019; 05. 20. pii: CJN.12651018. DOI: 10.2215/CJN.12651018. [Epub ahead of print].
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristics of Patients with Chronic Kidney Disease Associated with Marked Bradycardia
- Hyperkalemia in Chronic Kidney Disease
- Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: A Closer Look at Hyperkalemia
- Analysis of Hyperkalemia Inducing Factors in Patient with Chronic Renal Failure
- The gut-kidney connection in advanced chronic kidney disease