Anat Cell Biol.
2017 Jun;50(2):135-142. 10.5115/acb.2017.50.2.135.
Age-related change of Iba-1 immunoreactivity in the adult and aged gerbil spinal cord
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea. jhchoi@kangwon.ac.kr
- 2Department of Veterinary Internal Medicine and Geriatrics, College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea.
- 3Department of Anatomy and Cell Biology, College of Veterinary Medicine, and Research Institute for Veterinary Science, Seoul National University, Seoul, Korea.
- 4Department of Neurobiology, Kangwon National University School of Medicine, Chuncheon, Korea.
- KMID: 2451239
- DOI: http://doi.org/10.5115/acb.2017.50.2.135
Abstract
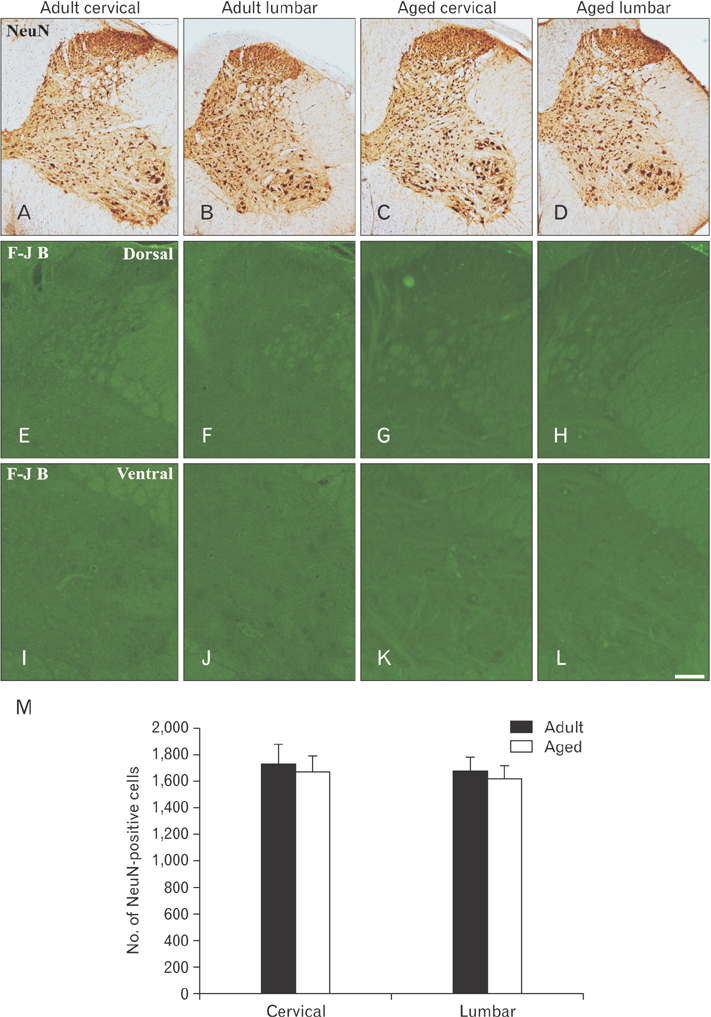
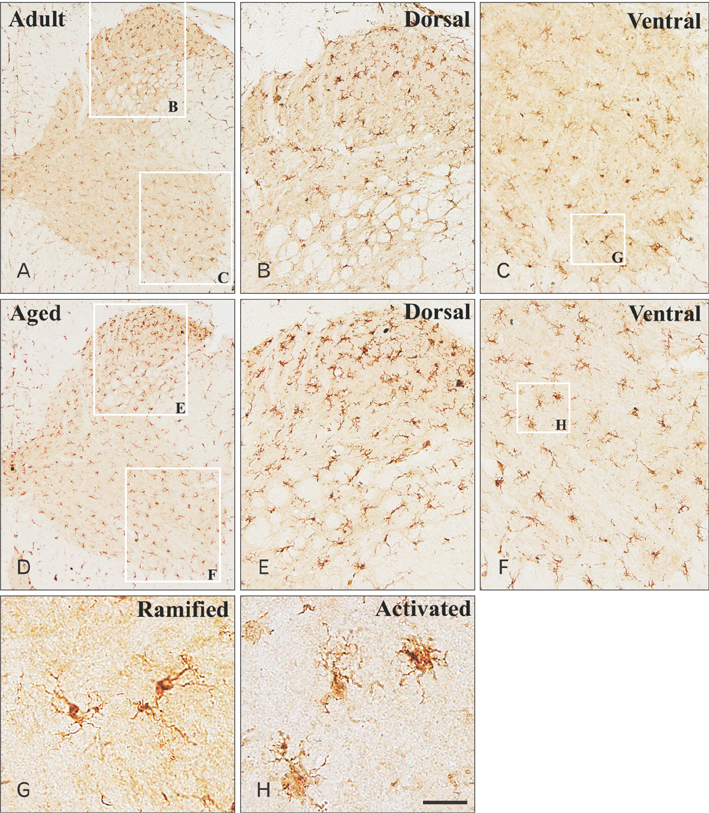
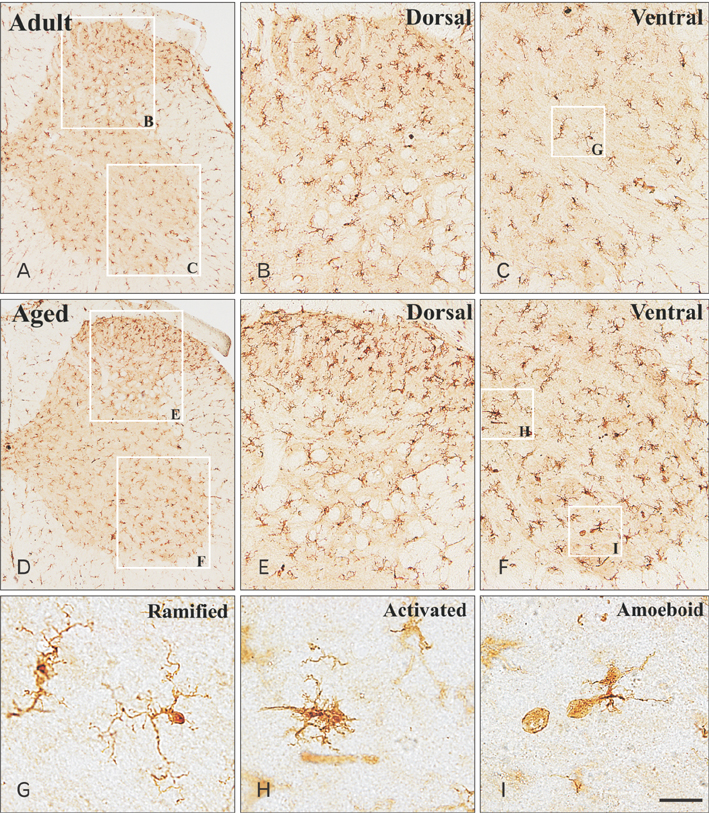
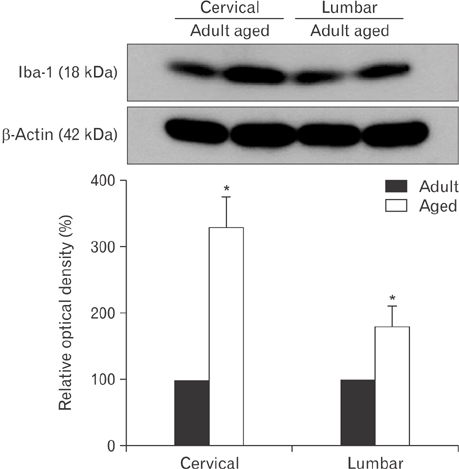
- In the present study, we examined change of ionized calcium-binding adapter molecule 1 (Iba-1) in the adult and aged gerbil spinal cords. Significant change of morphological feature and neuronal cell loss were not observed in both adult and aged spinal cords of gerbil after NeuN immunohistochemistry and Fluoro-Jade B histofluoresce staining. Iba-1-immunoreactive microglia broadly distributed in the spinal cord. Most of Iba-1-immunoreactive microglia showed ramified forms in the adult gerbil cervical and lumbar spinal cords. However, morphological changes of Iba-1-immunoreactive microglia were observed in the cervical and lumbar regions of the aged gerbil spinal cord. These microglia were showed a hypertrophied body with shortened swollen processes which was characteristic of activated microglia. In addition, Iba-1 protein level significantly higher in aged cervical and lumbar spinal cords than those in the adult gerbil. The present study showed an increase of activated forms of Iba-1-immunoreactive microglia and its protein level without marked changes in morphological features and neuronal loss in the aged spinal cord compared to those in the adult gerbil spinal cord. This result suggests that the increase of Iba-1 expression in the aged spinal cord may be closely associated with age-related changes in aged gerbil spinal cord.
Keyword
MeSH Terms
Figure
Reference
-
1. Streit WJ, Xue QS. The brain's aging immune system. Aging Dis. 2010; 1:254–261.2. Fu Y, Yu Y, Paxinos G, Watson C, Rusznák Z. Aging-dependent changes in the cellular composition of the mouse brain and spinal cord. Neuroscience. 2015; 290:406–420.3. Zhang B, Bailey WM, McVicar AL, Gensel JC. Age increases reactive oxygen species production in macrophages and potentiates oxidative damage after spinal cord injury. Neurobiol Aging. 2016; 47:157–167.4. Schmidt ML, Zhukareva V, Perl DP, Sheridan SK, Schuck T, Lee VM, Trojanowski JQ. Spinal cord neurofibrillary pathology in Alzheimer disease and Guam Parkinsonism-dementia complex. J Neuropathol Exp Neurol. 2001; 60:1075–1086.5. Seo JS, Leem YH, Lee KW, Kim SW, Lee JK, Han PL. Severe motor neuron degeneration in the spinal cord of the Tg2576 mouse model of Alzheimer disease. J Alzheimers Dis. 2010; 21:263–276.6. Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012; 122:1164–1171.7. Davis EJ, Foster TD, Thomas WE. Cellular forms and functions of brain microglia. Brain Res Bull. 1994; 34:73–78.8. Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998; 57:1–9.9. Aloisi F. Immune function of microglia. Glia. 2001; 36:165–179.10. Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene Iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996; 224:855–862.11. Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016; 64:300–316.12. Ton BH, Chen Q, Gaina G, Tucureanu C, Georgescu A, Strungaru C, Flonta ML, Sah D, Ristoiu V. Activation profile of dorsal root ganglia Iba-1 (+) macrophages varies with the type of lesion in rats. Acta Histochem. 2013; 115:840–850.13. Hwang IK, Yoo KY, Jung BK, Cho JH, Kim DH, Kang TC, Kwon YG, Kim YS, Won MH. Correlations between neuronal loss, decrease of memory, and decrease expression of brain-derived neurotrophic factor in the gerbil hippocampus during normal aging. Exp Neurol. 2006; 201:75–83.14. Rich ST. The Mongolian gerbil (Meriones unguiculatus) in research. Lab Anim Care. 1968; 18:Suppl. 235–243.15. Yoon DK, Hwang IK, Yoo KY, Lee YB, Lee JJ, Kim JH, Kang TC, Lee BH, Sohn HS, Won MH. Comparison of alpha-synuclein immunoreactivity and protein levels in ischemic hippocampal CA1 region between adult and aged gerbils and correlation with Cu,Zn-superoxide dismutase. Neurosci Res. 2006; 55:434–441.16. Cheal ML. The gerbil: a unique model for research on aging. Exp Aging Res. 1986; 12:3–21.17. Schmiedt RA. Effects of aging on potassium homeostasis and the endocochlear potential in the gerbil cochlea. Hear Res. 1996; 102:125–132.18. Meert TF, Vissers K, Geenen F, Kontinen VK. Functional role of exogenous administration of substance P in chronic constriction injury model of neuropathic pain in gerbils. Pharmacol Biochem Behav. 2003; 76:17–25.19. Lepre M, Evans RH, Olpe HR, Brugger F. The modulation of the monosynaptic reflex by substance P in the hemisected spinal cord preparation of the rat and gerbil. Neuroscience. 1993; 55:727–735.20. Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000; 874:123–130.21. Chung JY, Choi JH, Lee CH, Yoo KY, Won MH, Yoo DY, Kim DW, Choi SY, Youn HY, Moon SM, Hwang IK. Comparison of ionized calcium-binding adapter molecule 1-immunoreactive microglia in the spinal cord between young adult and aged dogs. Neurochem Res. 2010; 35:620–627.22. Lee HJ, Choi JH, Ahn JH, Lee CH, Yoo KY, Hwang IK, Kim JS, Kim C, Lee YL, Shin HC, Won MH. Comparison of GAD65 and 67 immunoreactivity in the lumbar spinal cord between young adult and aged dogs. Neurochem Res. 2011; 36:435–442.23. Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004; 45:208–212.24. Avignone E, Lepleux M, Angibaud J, Nägerl UV. Altered morphological dynamics of activated microglia after induction of status epilepticus. J Neuroinflammation. 2015; 12:202.25. Korzhevskiĭ DE, Lentsman MV, Kirik OV, Otellin VA. Morphological types of activated microglia in the hippocampus observed following transient total brain ischemia. Morfologiia. 2012; 142:30–33.26. Nakamura R, Nishimura T, Ochiai T, Nakada S, Nagatani M, Ogasawara H. Availability of a microglia and macrophage marker, iba-1, for differential diagnosis of spontaneous malignant reticuloses from astrocytomas in rats. J Toxicol Pathol. 2013; 26:55–60.27. Ahn JH, Shin MC, Park JH, Kim IH, Lee JC, Yan BC, Hwang IK, Moon SM, Ahn JY, Ohk TG, Lee TH, Cho JH, Shin HC, Won MH. Increased immunoreactivity of cFos in the spinal cord of the aged mouse and dog. Mol Med Rep. 2015; 11:1043–1048.28. Missale C, Govoni S, Castelletti L, Spano PF, Trabucchi M. Age related changes of enkephalin in rat spinal cord. Brain Res. 1983; 262:160–162.29. Ranson RN, Dodds AL, Smith MJ, Santer RM, Watson AH. Age-associated changes in the monoaminergic innervation of rat lumbosacral spinal cord. Brain Res. 2003; 972:149–158.30. Lozza FA, Chinchilla LA, Barbeito CG, Goya RG, Gimeno EJ, Portiansky EL. Changes in carbohydrate expression in the cervical spinal cord of rats during aging. Neuropathology. 2009; 29:258–262.31. Tan H, He J, Wang S, Hirata K, Yang Z, Kuraoka A, Kawabuchi M. Age-related NADPH-diaphorase positive bodies in the lumbosacral spinal cord of aged rats. Arch Histol Cytol. 2006; 69:297–310.32. Ahn JH, Choi JH, Kim JS, Lee HJ, Lee CH, Yoo KY, Hwang IK, Lee YL, Shin HC, Won MH. Comparison of immunoreactivities in 4-HNE and superoxide dismutases in the cervical and the lumbar spinal cord between adult and aged dogs. Exp Gerontol. 2011; 46:703–708.33. Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996; 19:312–318.34. Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998; 19:47–55.35. Hailer NP, Jarhult JD, Nitsch R. Resting microglial cells in vitro: analysis of morphology and adhesion molecule expression in organotypic hippocampal slice cultures. Glia. 1996; 18:319–331.36. Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006; 29:68–74.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spatial and temporal expression patterns of apoptosis-related genes in rat spinal cord during normal aging
- Comparison of alpha-synuclein immunoreactivity in the spinal cord between the adult and aged beagle dog
- Immunoreactivity of Androgen Receptor Protein in Sexually Dimorphic Spinal Motonucleus in Neonatal Male Rats
- Alterations of Glutamate and GABA Immunoreactivities in the Anterior Horn of the Ischemic Spinal Cord of the Rabbit
- Immunoreactivity of androgen receptor protein in sexually dimorphic spinal motonucleus in neonatal male rats