J Clin Neurol.
2019 Jan;15(1):20-26. 10.3988/jcn.2019.15.1.20.
Real-World Effectiveness of Disease-Modifying Therapies in Korean Patients with Relapsing Multiple Sclerosis
- Affiliations
-
- 1Department of Neurology, Research Institute and Hospital of National Cancer Center, Goyang, Korea. hojinkim@ncc.re.kr
- 2Department of Neurology, College of Medicine, Yeungnam University, Gyeongsan, Korea.
- 3Department of Neurology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 4Department of Neurology, Kosin University College of Medicine, Busan, Korea.
- KMID: 2451141
- DOI: http://doi.org/10.3988/jcn.2019.15.1.20
Abstract
- BACKGROUND AND PURPOSE
This study assessed the long-term outcomes of disease-modifying therapies (DMTs) in Korean multiple sclerosis (MS) patients treated in real-world clinical settings in Korea.
METHODS
We retrospectively evaluated the medical records of 160 patients with an initial diagnosis of clinically isolated syndrome or relapsing-remitting MS who were treated for at least 2 years. A status of 3 for no evidence of disease activity (NEDA3) was defined as no relapse, disability progression, or active lesions in annual magnetic resonance imaging (MRI) evaluations.
RESULTS
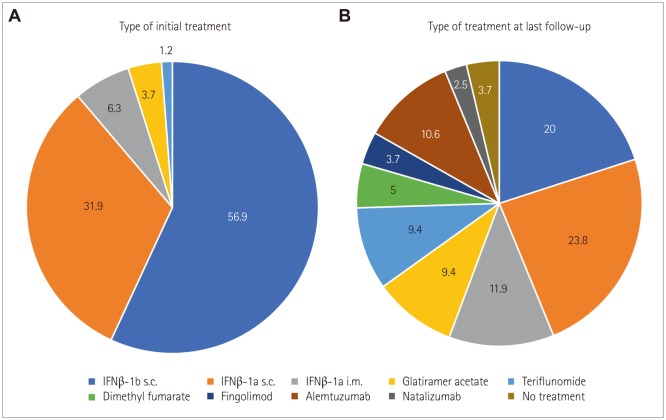
Patients who were initially treated with interferon β (n=152), glatiramer acetate (n=6), or teriflunomide (n=2) were included. The mean disease duration was 8.2 years. Compared to pretreatment, annualized relapse rates were significantly reduced after treatment [from 1.0±0.8 to 0.2±0.4 (mean±standard deviation), p < 0.001]. At the follow-up, 79 patients (49%) had changed their treatment regimen due to lack of efficacy (33%), side effects (14%), or other reasons (2%). Disability progression was observed in 18% of the patients over a mean treatment duration of 5.7 years. After 2 years, NEDA3 was observed in 38% of the patients. Loss of NEDA3 at 2 years was associated with long-term disability progression [odds ratio (OR)=17.975, p=0.003]. Poor response to first-line treatment was independently associated with a delay in treatment from disease onset (OR=1.238, p=0.049) and 10 or more brain lesions in the initial MRI (OR=3.648, p=0.047).
CONCLUSIONS
This study has provided real-world evidence that DMTs are effective in reducing disease activity and disability progression in Korean MS patients.
MeSH Terms
Figure
Reference
-
1. Kappos L, Kuhle J, Multanen J, Kremenchutzky M, Verdun di Cantogno E, Cornelisse P, et al. Factors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15. J Neurol Neurosurg Psychiatry. 2015; 86:1202–1207. PMID: 26374702.
Article2. University of California, San Francisco MS-EPIC Team. Cree BA, Gourraud PA, Oksenberg JR, Bevan C, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016; 80:499–510. PMID: 27464262.3. Jokubaitis VG, Spelman T, Kalincik T, Lorscheider J, Havrdova E, Horakova D, et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann Neurol. 2016; 80:89–100. PMID: 27145331.
Article4. Tintore M, Rovira À, Río J, Otero-Romero S, Arrambide G, Tur C, et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain. 2015; 138:1863–1874. PMID: 25902415.
Article5. Trojano M, Pellegrini F, Paolicelli D, Fuiani A, Zimatore GB, Tortorella C, et al. Real-life impact of early interferon beta therapy in relapsing multiple sclerosis. Ann Neurol. 2009; 66:513–520. PMID: 19847899.6. Kim NH, Kim HJ, Cheong HK, Kim BJ, Lee KH, Kim EH, et al. Prevalence of multiple sclerosis in Korea. Neurology. 2010; 75:1432–1438. PMID: 20956788.
Article7. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005; 58:840–846. PMID: 16283615.
Article8. O'Riordan JI, Thompson AJ, Kingsley DP, MacManus DG, Kendall BE, Rudge P, et al. The prognostic value of brain MRI in clinically isolated syndromes of the CNS. A 10-year follow-up. Brain. 1998; 121:495–503. PMID: 9549525.9. Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology. 2005; 64:1144–1151. PMID: 15824338.
Article10. Pachner AR, Steiner I. The multiple sclerosis severity score (MSSS) predicts disease severity over time. J Neurol Sci. 2009; 278:66–70. PMID: 19138773.
Article11. Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003; 126:770–782. PMID: 12615637.
Article12. Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014; 13:545–556. PMID: 24685276.
Article13. Kim SH, Huh SY, Kim W, Park MS, Ahn SW, Cho JY, et al. Clinical characteristics and outcome of multiple sclerosis in Korea: does multiple sclerosis in Korea really differ from that in the Caucasian populations? Mult Scler. 2013; 19:1493–1498. PMID: 23407702.
Article14. Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989; 112:133–146. PMID: 2917275.15. Piccolo L, Kumar G, Nakashima I, Misu T, Kong Y, Wakerley B, et al. Multiple sclerosis in Japan appears to be a milder disease compared to the UK. J Neurol. 2015; 262:831–836. PMID: 25605435.
Article16. Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol. 2015; 72:152–158. PMID: 25531931.
Article17. Nixon R, Bergvall N, Tomic D, Sfikas N, Cutter G, Giovannoni G. No evidence of disease activity: indirect comparisons of oral therapies for the treatment of relapsing-remitting multiple sclerosis. Adv Ther. 2014; 31:1134–1154. PMID: 25414048.
Article18. Uher T, Havrdova E, Sobisek L, Krasensky J, Vaneckova M, Seidl Z, et al. Is no evidence of disease activity an achievable goal in MS patients on intramuscular interferon beta-1a treatment over long-term follow-up? Mult Scler. 2017; 23:242–252. PMID: 27230790.
Article19. Río J, Rovira À, Tintoré M, Otero-Romero S, Comabella M, Vidal-Jordana Á, et al. Disability progression markers over 6–12 years in interferon-β-treated multiple sclerosis patients. Mult Scler. 2018; 24:322–330. PMID: 28287331.20. Erbayat Altay E, Fisher E, Jones SE, Hara-Cleaver C, Lee JC, Rudick RA. Reliability of classifying multiple sclerosis disease activity using magnetic resonance imaging in a multiple sclerosis clinic. JAMA Neurol. 2013; 70:338–344. PMID: 23599930.
Article21. Ransohoff RM, Hafler DA, Lucchinetti CF. Multiple sclerosis-a quiet revolution. Nat Rev Neurol. 2015; 11:134–142. PMID: 25686758.
Article22. Hupperts R, Ghazi-Visser L, Martins Silva A, Arvanitis M, Kuusisto H, Marhardt K, et al. The STAR study: a real-world, international, observational study of the safety and tolerability of, and adherence to, serum-free subcutaneous interferon β-1a in patients with relapsing multiple sclerosis. Clin Ther. 2014; 36:1946–1957. PMID: 24811754.
Article23. Carroll WM. Clinical trials of multiple sclerosis therapies: improvements to demonstrate long-term patient benefit. Mult Scler. 2009; 15:951–958. PMID: 19465446.
Article24. Freedman MS. Long-term follow-up of clinical trials of multiple sclerosis therapies. Neurology. 2011; 76:S26–S34.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Summary of Cladribine (from Development to Clinical and Practical Treatment)
- The Clinical Utility of the Ocrelizumab for the Patients with Multiple Sclerorsis
- Reduction of Disease Activity in Patient with Relapsing-Remitting Multiple Sclerosis after Switching to Teriflunomide from Interferon Beta
- Changes in the Multiple Sclerosis Treatment Paradigm. What Do We Do Now and What Were We Doing Before?
- Bruton’s Tyrosine Kinase Inhibitors for Multiple Sclerosis