Blood Res.
2018 Jun;53(2):138-144. 10.5045/br.2018.53.2.138.
Leukemia propagating cells in Philadelphia chromosome-positive ALL: a resistant phenotype with an adverse prognosis
- Affiliations
-
- 1Clinical Pathology Department, Hematology Unit, Mansoura Medical School, Mansoura University, Mansoura, Egypt.
- 2Internal Medicine Department, Specialized Medicine Hospital, Mansoura Medical School, Mansoura University, Mansoura, Egypt.
- 3Medical Oncology, Faculty of Medicine, Oncology Center, Mansoura University, Mansoura, Egypt. drmohamedawad@gmail.com
- KMID: 2451032
- DOI: http://doi.org/10.5045/br.2018.53.2.138
Abstract
- BACKGROUND
Targeted therapy has revolutionized the management of Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL); however, relapse still occurs because of the presence of quiescent stem cells, termed leukemia propagating cells (LPCs). This study aimed to assess the phenotypic diversity of LPCs in adult patients with Ph+ B-Acute ALL (B-ALL) and to assess its prognostic impact.
METHODS
Seventy adults with newly diagnosed Ph+ B-ALL were recruited at the Mansoura Oncology Center. Multiparameter flow cytometry studies of mononuclear blast cells for cluster of differentiation (CD)34, CD38, and CD58 were performed.
RESULTS
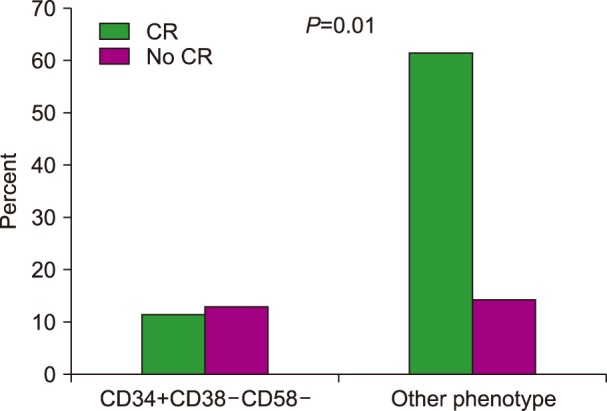
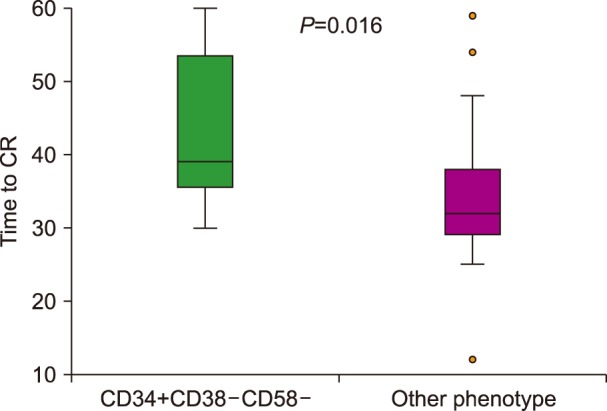
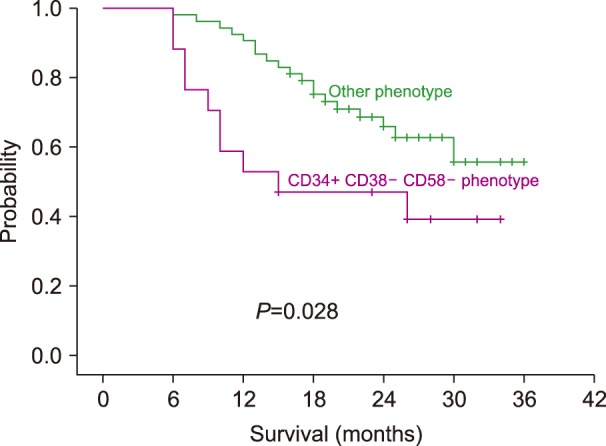
Seventeen patients had blasts with the pattern of LPCs (CD34+CD38−CD58−), while 53 cases had other diverse phenotypic patterns. The rate of complete response was significantly lower in patients with the LPC phenotype (47% vs. 81%, P=0.006). The median time to achieve a complete response was prolonged in patients with the CD34+CD38−CD58− phenotype (48 vs. 32 days, P=0.016). The three-year overall survival was significantly lower in patients with the CD34+CD38−CD58− phenotype (37% vs. 55% respectively, P=0.028). Multivariate analysis showed that the CD34+CD38− CD58− phenotype was an independent risk factor for overall survival.
CONCLUSION
The presence of CD34+CD38−CD58− LPCs at diagnosis allows rapid identification of higher risk patients. Risk stratification of these patients is needed to further guide therapy and develop effective LPCs-targeted therapy to improve treatment outcome.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Comparative evaluation of the developed targeted RNA sequencing system and a commercialized test panel
Young Eun Lee, Ju Heon Park, Ha Jin Lim, Hye Ran Kim, Jong Hee Shin, Myung Geun Shin
Blood Res. 2022;57(3):235-238. doi: 10.5045/br.2022.2022095.
Reference
-
1. Ottmann OG, Pfeifer H. Management of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). Hematology Am Soc Hematol Educ Program. 2009; 371–381. PMID: 20008223.
Article2. Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011; 29:532–543. PMID: 21220592.
Article3. Gökbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Semin Hematol. 2009; 46:64–75. PMID: 19100369.
Article4. Cobaleda C, Gutiérrez-Cianca N, Pérez-Losada J, et al. A primitive hematopoietic cell is the target for the leukemic transformation in human Philadelphia-positive acute lymphoblastic leukemia. Blood. 2000; 95:1007–1013. PMID: 10648416.
Article5. Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994; 367:645–648. PMID: 7509044.
Article6. Eppert K, Takenaka K, Lechman ER, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011; 17:1086–1093. PMID: 21873988.
Article7. Kong Y, Yoshida S, Saito Y, et al. CD34+CD38+CD19+ as well as CD34+CD38−CD19+ cells are leukemia-initiating cells with self-renewal capacity in human B-precursor ALL. Leukemia. 2008; 22:1207–1213. PMID: 18418410.
Article8. Long J, Liu S, Li K, Zhou X, Zhang P, Zou L. High proportion of CD34+/CD38−cells is positively correlated with poor prognosis in newly diagnosed childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2014; 55:611–617. PMID: 23706103.
Article9. Wang JH, Smolyar A, Tan K, et al. Structure of a heterophilic adhesion complex between the human CD2 and CD58 (LFA-3) counterreceptors. Cell. 1999; 97:791–803. PMID: 10380930.
Article10. Archimbaud E, Thomas X, Campos L, Magaud JP, Doré JF, Fiere D. Expression of surface adhesion molecules CD54 (ICAM-1) and CD58 (LFA-3) in adult acute leukemia: relationship with initial characteristics and prognosis. Leukemia. 1992; 6:265–271. PMID: 1375302.11. Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004; 103:4396–4407. PMID: 14551133.
Article12. Shaffer LG, McGowan-Jordan J, Schmid M. ISCN 2013: An International System for Human Cytogenetic Nomenclature (2013). Basel, Switzerland: S. Karger;2013.13. Kong Y, Chang YJ, Liu YR, et al. CD34(+)CD38(−)CD58(−) cells are leukemia-propagating cells in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2014; 28:2398–2401. PMID: 25135692.
Article14. le Viseur C, Hotfilder M, Bomken S, et al. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008; 14:47–58. PMID: 18598943.
Article15. Aoki Y, Watanabe T, Saito Y, et al. Identification of CD34+ and CD34− leukemia-initiating cells in MLL-rearranged human acute lymphoblastic leukemia. Blood. 2015; 125:967–980. PMID: 25538041.
Article16. Kong Y, Xu LP, Liu YR, et al. Presence of CD34(+)CD38(−)CD58(−) leukemia-propagating cells at diagnosis identifies patients at high risk of relapse with Ph chromosome-positive ALL after all-ohematopoietic SCT. Bone Marrow Transplant. 2015; 50:348–353. PMID: 25486581.
Article17. Gunjal P, Pedziwiatr D, Ismail AA, Kakar SS, Ratajczak MZ. An emerging question about putative cancer stem cells in established cell lines-are they true stem cells or a fluctuating cell phenotype? J Cancer Stem Cell Res. 2015; 3:pii:e1004.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Complete remission of philadelphia chromosome-positive acute myeloid leukemia with imatinib mesylate
- A Case of Myelocytic Leukemia with Philadelphia Chromosome
- Two Cases of Chronic Myeloid Leukemia in Lymphoid Blast Phase Presented as Philadelphia-Positive Acute Lymphoblastic Leukemia
- A Novel Four-Way Translocation t(5;9;22;18)(q31;q34;q11.2;q21) in a Patient with Chronic Myelogenous Leukemia
- A case of chronic myeloid leukemia with features of essential thrombocythemia in peripheral blood and bone marrow