Ann Lab Med.
2019 Nov;39(6):596-598. 10.3343/alm.2019.39.6.596.
First Isolation of Mycobacterium virginiense From a Human Pulmonary Specimen
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. pmhhj77@gmail.com
- 2Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2450960
- DOI: http://doi.org/10.3343/alm.2019.39.6.596
Abstract
- No abstract available.
MeSH Terms
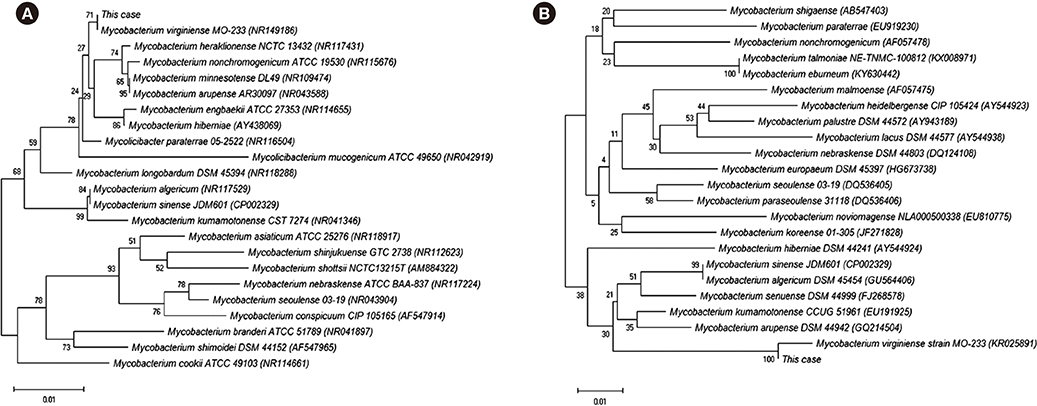
Figure
Reference
-
1. Vasireddy R, Vasireddy S, Brown-Elliott BA, Wengenack NL, Eke UA, Benwill JL, et al. Mycobacterium arupense, Mycobacterium heraklionense, and a newly proposed species, “Mycobacterium virginiense” sp. nov., but not Mycobacterium nonchromogenicum, as species of the Mycobacterium terrae complex causing tenosynovitis and osteomyelitis. J Clin Microbiol. 2016; 54:1340–1351.2. Vasireddy R, Vasireddy S, Brown-Elliott BA, Wengenack NL, Eke UA, Benwill JL, et al. Correction for Vasireddy et al., Mycobacterium arupense, Mycobacterium heraklionense, and a newly proposed species, “Mycobacterium virginiense” sp. nov., but not Mycobacterium nonchromogenicum, as species of the Mycobacterium terrae complex causing tenosynovitis and osteomyelitis. J Clin Microbiol. 2017; 55:985.3. Janda JM. Taxonomic update on proposed nomenclature and classification changes for bacteria of medical importance, 2016. Diagn Microbiol Infect Dis. 2017; 88:100–105.
Article4. Ito T, Maruyama F, Sawai K, Nozaki K, Otsu K, Ohya K. Draft genome sequence of Mycobacterium virginiense Strain GF75, isolated from the mud of a swine farm in Japan. Genome Announc. 2018; 6:e00362-18.
Article5. Park HT, Park HE, Jung YH, Yoo HS. An ISMap02-like insertion sequence in Mycobacterium spp. interferes with specific detection of Mycobacterium avium subsp. paratuberculosis. Vet Microbiol. 2018; 216:1–6.6. CLSI. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing; approved guideline. CLSI document No. MM18-A. Wayne, PA: Clinical and Laboratory Standards Institute;2008.7. CLSI. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. CLSI document No. M24-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2011.8. Smith DS, Lindholm-Levy P, Huitt GA, Heifets LB, Cook JL. Mycobacterium terrae: case reports, literature review, and in vitro antibiotic susceptibility testing. Clin Infect Dis. 2000; 30:444–453.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isolation of Mycobacterium lentiflavum from a Patient with a Lung Destroyed by Tuberculosis
- Diagnosis and Treatment of Nontuberculous Mycobacterial Lung Disease
- Pulmonary Infection Caused by Mycobacterium neoaurum: The First Case in Korea
- Mycobacterium Szulgai Pulmonary Infection: Case Report of an Uncommon Pathogen in Korea
- Mycobacterium kansasii Pulmonary Diseases in Korea