Yonsei Med J.
2015 May;56(3):785-792. 10.3349/ymj.2015.56.3.785.
Triglyceride Is a Useful Surrogate Marker for Insulin Resistance in Korean Women with Polycystic Ovary Syndrome
- Affiliations
-
- 1Department of Obstetrics and Gynecology, School of Medicine, Ewha Womans University, Seoul, Korea. ogjeong@ewha.ac.kr
- 2Department of Obstetrics and Gynecology, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2450354
- DOI: http://doi.org/10.3349/ymj.2015.56.3.785
Abstract
- PURPOSE
To evaluate lipid profiles and liver enzymes as surrogate markers used for recognizing insulin resistance in Korean women with polycystic ovary syndrome (PCOS).
MATERIALS AND METHODS
458 women with PCOS were divided into two groups: non-obese with a body mass index (BMI)<25.0 kg/m2 and obese with a BMI> or =25.0 kg/m2. Anthropometric measures and blood sampling for hormone assay, liver enzymes, lipid profiles and 75 g oral glucose tolerance test were performed. Insulin resistance was defined as homeostasis model assessment of insulin resistance (HOMA-IR)> or =2.5. Areas under the receiver operating characteristic (ROC) curves were used to compare the power of serum markers. Multiple linear regression analysis was used to evaluate the contribution of each confounding factor for HOMA-IR.
RESULTS
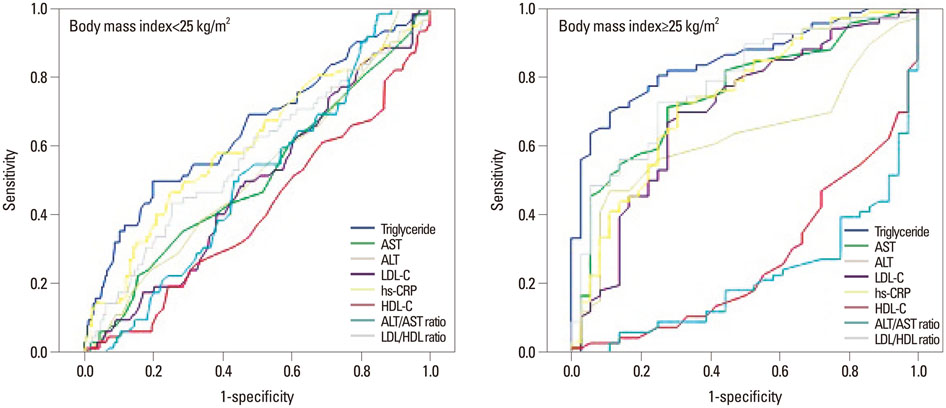
In non-obese and obese groups, the ROC curve analyses demonstrated that the best marker for insulin resistance was triglyceride (TG), with the areas under the ROC curve of 0.617 and 0.837, respectively. Low-density lipoprotein cholesterol (LDL-C) was the significant marker for insulin resistance with areas under the ROC curve of 0.698 in obese group, but not significant in non-obese group. TG and LDL-C were significantly associated with HOMA-IR in both non-obese and obese PCOS women by multiple linear regression analysis. The optimal cut-off points of TG> or =68.5 was a marker for predicting insulin resistance in non-obese PCOS patients and TG> or =100.5 in obese group.
CONCLUSION
TG can be used as a useful marker for insulin resistance in Korean women with PCOS, especially for obese patients.
MeSH Terms
-
Adult
Asian Continental Ancestry Group/ethnology
Biological Markers/blood
Body Mass Index
Cholesterol, LDL/blood
Female
Glucose Tolerance Test
Humans
Insulin/blood
Insulin Resistance/ethnology/*physiology
Lipids/blood
Obesity/*blood/ethnology
Polycystic Ovary Syndrome/*blood/ethnology
ROC Curve
Regression Analysis
Republic of Korea/epidemiology
Triglycerides/*blood
Biological Markers
Cholesterol, LDL
Insulin
Lipids
Triglycerides
Figure
Cited by 2 articles
-
Triglycerides to High-Density Lipoprotein Cholesterol Ratio Can Predict Impaired Glucose Tolerance in Young Women with Polycystic Ovary Syndrome
Do Kyeong Song, Hyejin Lee, Yeon-Ah Sung, Jee-Young Oh
Yonsei Med J. 2016;57(6):1404-1411. doi: 10.3349/ymj.2016.57.6.1404.Characteristics Predictive for a Successful Switch from Insulin Analogue Therapy to Oral Hypoglycemic Agents in Patients with Type 2 Diabetes
Gyuri Kim, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Yonsei Med J. 2016;57(6):1395-1403. doi: 10.3349/ymj.2016.57.6.1395.
Reference
-
1. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000; 106:453–458.
Article2. Laws A, Reaven GM. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J Intern Med. 1992; 231:25–30.
Article3. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.
Article4. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000; 85:2402–2410.
Article5. Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000; 23:57–63.
Article6. Steinvil A, Shapira I, Ben-Bassat OK, Cohen M, Vered Y, Berliner S, et al. The association of higher levels of within-normal-limits liver enzymes and the prevalence of the metabolic syndrome. Cardiovasc Diabetol. 2010; 9:30.
Article7. Doi Y, Kubo M, Yonemoto K, Ninomiya T, Iwase M, Tanizaki Y, et al. Liver enzymes as a predictor for incident diabetes in a Japanese population: the Hisayama study. Obesity (Silver Spring). 2007; 15:1841–1850.
Article8. Sookoian S, Pirola CJ. Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol. 2012; 18:3775–3781.
Article9. Xu Y, Bi YF, Xu M, Huang Y, Lu WY, Gu YF, et al. Cross-sectional and longitudinal association of serum alanine aminotransaminase and γ-glutamyltransferase with metabolic syndrome in middle-aged and elderly Chinese people. J Diabetes. 2011; 3:38–47.
Article10. Monami M, Bardini G, Lamanna C, Pala L, Cresci B, Francesconi P, et al. Liver enzymes and risk of diabetes and cardiovascular disease: results of the Firenze Bagno a Ripoli (FIBAR) study. Metabolism. 2008; 57:387–392.
Article11. Tousoulis D, Papageorgiou N, Androulakis E, Siasos G, Latsios G, Tentolouris K, et al. Diabetes mellitus-associated vascular impairment: novel circulating biomarkers and therapeutic approaches. J Am Coll Cardiol. 2013; 62:667–676.12. Pfützner A, Forst T. High-sensitivity C-reactive protein as cardiovascular risk marker in patients with diabetes mellitus. Diabetes Technol Ther. 2006; 8:28–36.
Article13. Kimberly MM, Cooper GR, Myers GL. An overview of inflammatory markers in type 2 diabetes from the perspective of the clinical chemist. Diabetes Technol Ther. 2006; 8:37–44.
Article14. Nakagami H, Kaneda Y, Ogihara T, Morishita R. Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Curr Diabetes Rev. 2005; 1:59–63.
Article15. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004; 19:41–47.16. Park SH, Lee WY, Rhee EJ, Jeon WK, Kim BI, Ryu SH, et al. Relative risks of the metabolic syndrome according to the degree of insulin resistance in apparently healthy Korean adults. Clin Sci (Lond). 2005; 108:553–559.
Article17. Taniguchi A, Fukushima M, Sakai M, Kataoka K, Miwa K, Nagata I, et al. Insulin-sensitive and insulin-resistant variants in nonobese Japanese type 2 diabetic patients. The role of triglycerides in insulin resistance. Diabetes Care. 1999; 22:2100–2101.
Article18. Kawamoto R, Kohara K, Kusunoki T, Tabara Y, Abe M, Miki T. Alanine aminotransferase/aspartate aminotransferase ratio is the best surrogate marker for insulin resistance in non-obese Japanese adults. Cardiovasc Diabetol. 2012; 11:117.
Article19. Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM. Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care. 2000; 23:171–175.
Article20. Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, et al. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes. 1998; 47:1643–1649.
Article21. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004; 27:1487–1495.
Article22. Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001; 138:38–44.
Article23. Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003; 52:908–915.
Article24. Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005; 90:1929–1935.
Article25. Kim-Dorner SJ, Deuster PA, Zeno SA, Remaley AT, Poth M. Should triglycerides and the triglycerides to high-density lipoprotein cholesterol ratio be used as surrogates for insulin resistance. Metabolism. 2010; 59:299–304.
Article26. Kannel WB, Vasan RS, Keyes MJ, Sullivan LM, Robins SJ. Usefulness of the triglyceride-high-density lipoprotein versus the cholesterol-high-density lipoprotein ratio for predicting insulin resistance and cardiometabolic risk (from the Framingham Offspring Cohort). Am J Cardiol. 2008; 101:497–501.
Article27. Tamada M, Makita S, Abiko A, Naganuma Y, Nagai M, Nakamura M. Low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a useful marker for early-stage carotid atherosclerosis. Metabolism. 2010; 59:653–657.
Article28. Kimm H, Lee SW, Lee HS, Shim KW, Cho CY, Yun JE, et al. Associations between lipid measures and metabolic syndrome, insulin resistance and adiponectin. - Usefulness of lipid ratios in Korean men and women -. Circ J. 2010; 74:931–937.
Article29. Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes. 1993; 42:833–842.
Article30. Yi KH, Hwang JS, Kim EY, Lee SH, Kim DH, Lim JS. Prevalence of insulin resistance and cardiometabolic risk in Korean children and adolescents: a population-based study. Diabetes Res Clin Pract. 2014; 103:106–113.
Article31. Lee S, Choi S, Kim HJ, Chung YS, Lee KW, Lee HC, et al. Cutoff values of surrogate measures of insulin resistance for metabolic syndrome in Korean non-diabetic adults. J Korean Med Sci. 2006; 21:695–700.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polycystic Ovary Syndrome in Adolescence. Role of Insulin Resistance
- Triglyceride and glucose index for identifying abnormal insulin sensitivity in women with polycystic ovary syndrome
- Insulin Resistance in Polycystic Ovary Syndrome
- Polycystic Ovary Syndrome in Korean Women: Clinical Characteristics and Diagnostic Criteria
- Obesity and Polycystic Ovary Syndrome