Int J Thyroidol.
2019 May;12(1):35-43. 10.11106/ijt.2019.12.1.35.
Ultrasonographic Characteristics of the Hyperfunctioning Thyroid Nodule and Predictive Factors for Thyroid Stimulating Hormone Suppression
- Affiliations
-
- 1Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea.
- 2Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea. reality0719@kangwon.ac.kr
- KMID: 2449064
- DOI: http://doi.org/10.11106/ijt.2019.12.1.35
Abstract
- BACKGROUND AND OBJECTIVES
Thyroid scan is a good tool for diagnosis of hyperfunctioning thyroid nodules (HNs), however it has been limited in use in a primary clinical practice, because of its inconvenience and low accessibility. This study aimed to analyze ultrasonographic (US) characteristics of HNs and to predict HNs by US.
MATERIALS AND METHODS
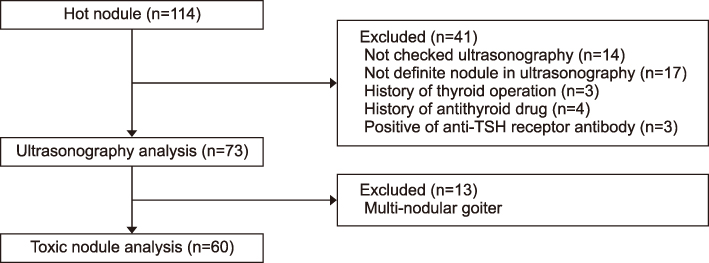
We included 114 patients who exhibited results of "˜hot' nodule in the thyroid scan from 2008 to 2017. Analysis for US characteristics included 73 patients without unclear US images and other inevitable reasons. We compared US characteristics of HNs with cold nodules that showed "cold" in the thyroid scan. Additionally, we compared US characteristics of HNs between suppressed thyroid-stimulating hormone (TSH) (<0.25 uIU/mL) or normal TSH, and analysis receiver operating characteristics (ROC) curve for prediction of suppressed TSH among HNs.
RESULTS
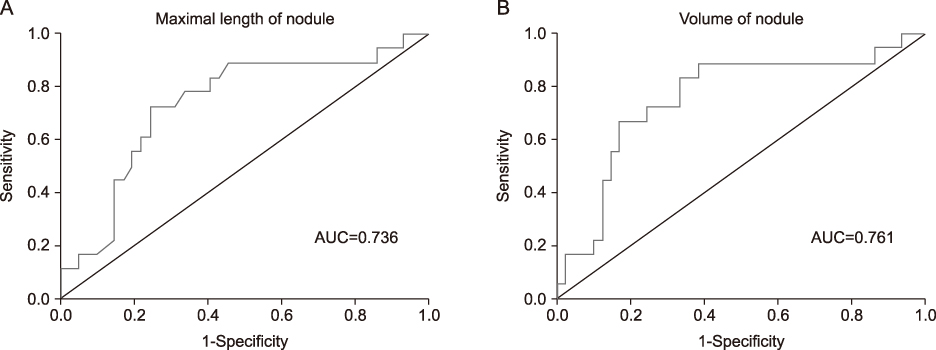
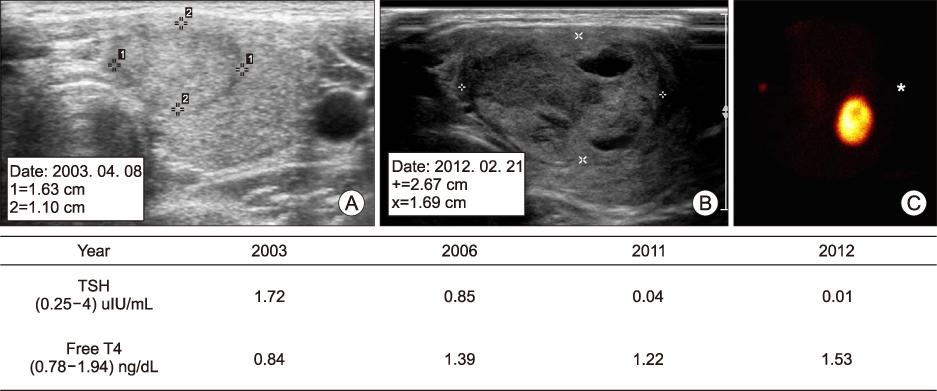
The HNs showed more partially cystic nodule, isoechoic echogenicity, hypervascularity and presence of halo in the US finding than the cold nodule. In subgroup analysis of nodules with TSH suppression among HNs, the TSH suppression nodules was lager in max size and volume than the normal TSH nodules. In ROC analyses for prediction of the TSH suppression among HNs, area under receiver operating characteristics curves was 0.736 in max size, 0.761 in volume.
CONCLUSION
HNs showed more frequently partially cystic contents, isoechoic echogenicity, hypervascularity, and peripheral halo sign in US finding. Thyroid nodule size and volume were associated with suppressed TSH level of HNs, and optimal cutoff levels for prediction of TSH suppression among HNs were 2.6 cm and 1.13 cm3, respectively.
MeSH Terms
Figure
Reference
-
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26(1):1–133.
Article2. Yi KH, Lee EK, Kang HC, Koh Y, Kim SW, Kim IJ, et al. 2016 revised Korean Thyroid Association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol. 2016; 9(2):59–126.
Article3. Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014; 81:Suppl 1. 1–122.
Article4. Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007; 36(3):707–735.
Article5. Kim WJ, Kim JH, Park DW, Lee CB, Park YS, Kim DS, et al. Prevalence of thyroid nodules detected by ultrasonography in adults for health check-ups and analysis of fine needle aspiration cytology. J Korean Endocr Soc. 2008; 23(6):413–419.
Article6. Ianni F, Perotti G, Prete A, Paragliola RM, Ricciato MP, Carrozza C, et al. Thyroid scintigraphy: An old tool is still the gold standard for an effective diagnosis of autonomously functioning thyroid nodules. J Endocrinol Invest. 2013; 36(4):233–236.7. Lee ES, Kim JH, Na DG, Paeng JC, Min HS, Choi SH, et al. Hyperfunction thyroid nodules: Their risk for becoming or being associated with thyroid cancers. Korean J Radiol. 2013; 14(4):643–652.
Article8. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17(3):370–395.
Article9. Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009; 19(11):1159–1165.
Article10. Moon WJ, Kwag HJ, Na DG. Are there any specific ultrasound findings of nodular hyperplasia (“leave me alone” lesion) to differentiate it from follicular adenoma. Acta Radiol. 2009; 50(4):383–388.
Article11. Mizukami Y, Michigishi T, Nonomura A, Yokoyama K, Noguchi M, Hashimoto T, et al. Autonomously functioning (hot) nodule of the thyroid gland. A clinical and histopathologic study of 17 cases. Am J Clin Pathol. 1994; 101(1):29–35.
Article12. Ruhlmann M, Stebner V, Gorges R, Farahati J, Simon D, Bockisch A, et al. Diagnosis of hyperfunctional thyroid nodules: impact of US-elastography. Nuklearmedizin. 2014; 53(5):173–177.13. Zandieh S, Muin D, Bernt R, Hittmair K, Haller J, Hergan K. Characteristics of incidentally found thyroid nodules in computed tomography: comparison with thyroid scintigraphy. BMC Med Imaging. 2017; 17(1):8.
Article14. Burch HB, Shakir F, Fitzsimmons TR, Jaques DP, Shriver CD. Diagnosis and management of the autonomously functioning thyroid nodule: the Walter Reed Army Medical Center experience, 1975-1996. Thyroid. 1998; 8(10):871–880.
Article15. Iwata M, Kasagi K, Hatabu H, Misaki T, Iida Y, Fujita T, et al. Causes of appearance of scintigraphic hot areas on thyroid scintigraphy analyzed with clinical features and comparative ultrasonographic findings. Ann Nucl Med. 2002; 16(4):279–287.
Article16. Kim JH, Na GJ, Kim KW, Ko HJ, Jeon SW, Kim YJ, et al. Papillary thyroid carcinoma manifesting as a autonomously functioning thyroid nodule. Endocrinol Metab. 2012; 27(1):59–62.
Article17. Kong SH, Lee SY, Yang YS, Moon JH. Papillary thyroid carcinoma presented as a hot nodule with hyperthyroidism. Int J Thyroidol. 2016; 9(1):47–50.
Article18. Soelberg KK, Grupe P, Boel-Jorgensen H, Jorgensen PH, Fast S, Nielsen VE, et al. Substantial interobserver variation of thyroid volume and function by visual evaluation of thyroid (99m)Tc scintigraphy. Dan Med J. 2014; 61(2):A4768.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Papillary Thyroid Carcinoma Presented as a Hot Nodule with Hyperthyroidism
- Hyperfunction Thyroid Nodules: Their Risk for Becoming or Being Associated with Thyroid Cancers
- The comparisons in ultrasonographic evaluation with radioisotope thyroid scanning in thyroid nodule
- Significance of Surgical Treatment for Toxic Thyroid Nodule
- Evaluation and Management of Bone Health in Patients with Thyroid Diseases: a Position Statement from the Korean Thyroid Association