Investig Clin Urol.
2019 May;60(3):195-201. 10.4111/icu.2019.60.3.195.
Use of docetaxel plus androgen deprivation therapy for metastatic hormone-sensitive prostate cancer in Korean patients: A retrospective study
- Affiliations
-
- 1Department of Urology, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea.
- 2Department of Urology, Urological Cancer Center, National Cancer Center, Goyang, Korea.
- 3Department of Urology, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea.
- 4Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. cskim@amc.seoul.kr
- KMID: 2444274
- DOI: http://doi.org/10.4111/icu.2019.60.3.195
Abstract
- PURPOSE
We aimed to evaluate the efficacy and safety of the use of docetaxel plus androgen deprivation therapy (ADT) for metastatic hormone-sensitive prostate cancer (mHSPC) in Korean patients.
MATERIALS AND METHODS
This study was conducted retrospectively. In total, 61 Korean patients with mHSPC who used docetaxel plus ADT were identified from medical records. Patients received docetaxel plus ADT at a dose of 75 mg/m2 every 3 weeks for 6 cycles. We evaluated prostate-specific antigen (PSA) response, PSA progression, progression to castration-resistant prostate cancer (CRPC), clinical progression, and adverse events.
RESULTS
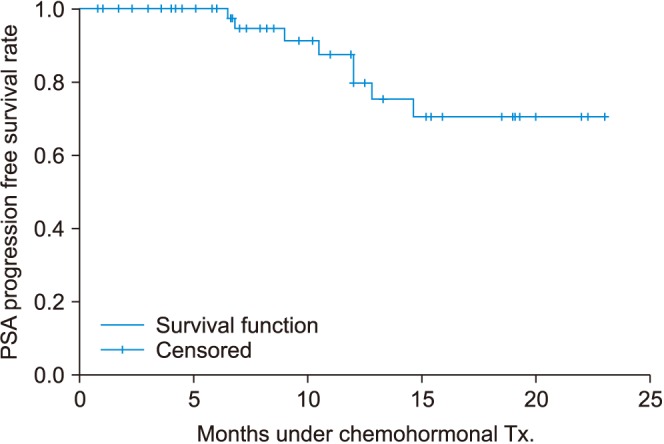
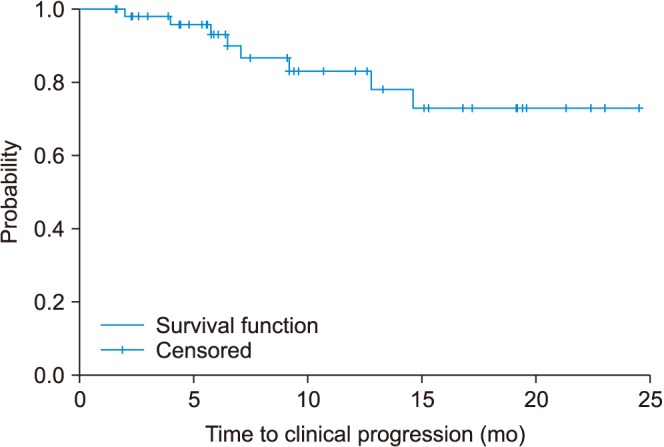
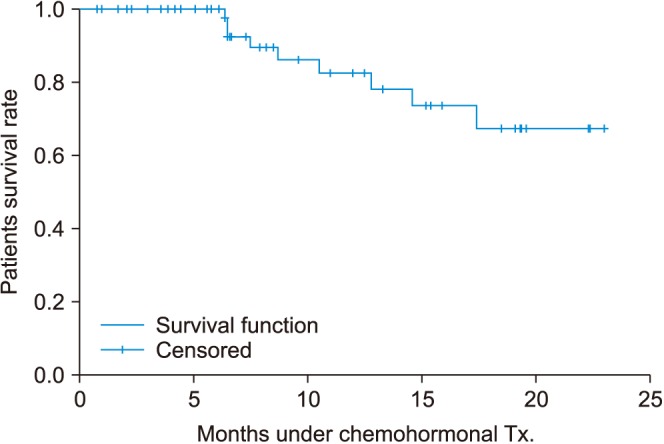
Most of the patients had high volume disease (98.3%) and 83.6% had a Gleason score of 8 or higher. The median PSA level at the start of ADT was 131.4 ng/mL. The percentage of patients whose PSA levels decreased to less than 0.2 ng/mL at 3, 6, and 12 months were 28.3%, 41.0%, and 45.0%, respectively. During a median of 12.0 months after treatment, PSA progression occurred in 13.3% of patients. Clinical progression and progression to CRPC were observed in 15.1% and 14.8%, respectively. Neutropenia grade ≥3 and febrile neutropenia occurred in 63.5% and 11.5%, respectively.
CONCLUSIONS
Comparing our findings with those of the prior chemohormonal therapy versus androgen ablation randomized trial for extensive disease in prostate cancer (CHAARTED) study, in Korean patients, the use of docetaxel plus ADT for mHSPC showed similar results for early oncologic outcomes including PSA response and time to clinical progression. However, we observed a higher rate of adverse events, which should be considered seriously.
Keyword
MeSH Terms
Figure
Reference
-
1. Roehrborn CG, Black LK. The economic burden of prostate cancer. BJU Int. 2011; 108:806–813. PMID: 21884356.
Article2. Beltran H, Beer TM, Carducci MA, de Bono J, Gleave M, Hussain M, et al. New therapies for castration-resistant prostate cancer: efficacy and safety. Eur Urol. 2011; 60:279–290. PMID: 21592649.
Article3. Botrel TE, Clark O, Pompeo AC, Bretas FF, Sadi MV, Ferreira U, et al. Immunotherapy with Sipuleucel-T (APC8015) in patients with metastatic castration-refractory prostate cancer (mCRPC): a systematic review and meta-analysis. Int Braz J Urol. 2012; 38:717–727. PMID: 23302410.
Article4. Huggins C, Stevens RE Jr, Hodges CV. Studies on prostatic cancer: II. the effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941; 43:209–223.5. Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002; 52:154–179. PMID: 12018929.
Article6. Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005; 23:8253–8261. PMID: 16278481.
Article7. Nelson PS. Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancer. J Clin Oncol. 2012; 30:644–646. PMID: 22184375.
Article8. Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014; 371:1028–1038. PMID: 25184630.
Article9. Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011; 17:5913–5925. PMID: 21807635.
Article10. Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006; 66:2815–2825. PMID: 16510604.
Article11. Ahmed M, Li LC. Adaptation and clonal selection models of castration-resistant prostate cancer: current perspective. Int J Urol. 2013; 20:362–371. PMID: 23163774.
Article12. Millikan RE, Wen S, Pagliaro LC, Brown MA, Moomey B, Do KA, et al. Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer. J Clin Oncol. 2008; 26:5936–5942. PMID: 19029421.
Article13. Wang J, Halford S, Rigg A, Roylance R, Lynch M, Waxman J. Adjuvant mitozantrone chemotherapy in advanced prostate cancer. BJU Int. 2000; 86:675–680. PMID: 11069375.
Article14. Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Esterni B, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013; 14:149–158. PMID: 23306100.15. Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015; 373:737–746. PMID: 26244877.
Article16. James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. ; STAMPEDE investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016; 387:1163–1177. PMID: 26719232.17. Jeong IG, Dajani D, Verghese M, Hwang J, Cho YM, Hong JH, et al. Differences in the aggressiveness of prostate cancer among Korean, Caucasian, and African American men: a retrospective cohort study of radical prostatectomy. Urol Oncol. 2016; 34:3.e9–3.e14.
Article18. Choi SK, Song C, Shim M, Min GE, Park J, Jeong IG, et al. Prevalence of high-grade or insignificant prostate cancer in Korean men with prostate-specific antigen levels of 3.0-4.0 ng/mL. Urology. 2015; 85:610–615. PMID: 25586476.19. Song C, Ro JY, Lee MS, Hong SJ, Chung BH, Choi HY, et al. Prostate cancer in Korean men exhibits poor differentiation and is adversely related to prognosis after radical prostatectomy. Urology. 2006; 68:820–824. PMID: 17070360.
Article21. Monn MF, Tatem AJ, Cheng L. Prevalence and management of prostate cancer among East Asian men: current trends and future perspectives. Urol Oncol. 2016; 34:58.e1–58.e9.
Article22. Zhu Y, Wang HK, Qu YY, Ye DW. Prostate cancer in East Asia: evolving trend over the last decade. Asian J Androl. 2015; 17:48–57. PMID: 25080928.
Article23. Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999; 17:3461–3467. PMID: 10550143.
Article24. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.25. Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED Trial. J Clin Oncol. 2018; 36:1080–1087. PMID: 29384722.
Article26. Tanaka N, Nishimura K, Okajima E, Ina K, Ogawa O, Nagata H, et al. The efficacy and safety of docetaxel-based chemotherapy combined with dexamethasone 1 mg daily oral administration: JMTO Pca 10-01 phase II trial. Jpn J Clin Oncol. 2017; 47:247–251. PMID: 28042138.
Article27. Lavoie JM, Zou K, Khalaf D, Eigl BJ, Kollmannsberger CK, Vergidis J, et al. Real-world experience with docetaxel for castration-sensitive prostate cancer from a population-based analysis. J Clinl Oncol. 2018; 36(6 suppl):297.
Article28. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines1). Prostate Cancer (Version 1.2016) [Internet]. Plymouth Meeting: National Comprehensive Cancer Network;cited 2017 Nov 11. Available from: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.29. Parker C, Gillessen S, Heidenreich A, Horwich A. ESMO Guidelines Committee. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015; 26(Suppl 5):v69–v77. PMID: 26205393.
Article30. Gillessen S, Omlin A, Attard G, de Bono JS, Efstathiou E, Fizazi K, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol. 2015; 26:1589–1604. PMID: 26041764.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Systemic Therapies for Metastatic Castration-Resistant Prostate Cancer: An Updated Review
- Current Concepts in Androgen Deprivation Therapy
- Effectiveness of Adding Docetaxel to Androgen Deprivation Therapy for Metastatic Hormone-Sensitive Prostate Cancer in Korean Real-World Practice
- Quality of Life in Prostate Cancer Patient Undergoing Androgen Deprivation Therapy