Korean J Physiol Pharmacol.
2019 Jan;23(1):55-62. 10.4196/kjpp.2019.23.1.55.
Pharmacological evaluation of HM41322, a novel SGLT1/2 dual inhibitor, in vitro and in vivo
- Affiliations
-
- 1Hanmi Research Center, Hanmi Pharmaceutical Co., Ltd, Hwaseong 18469, Korea.
- 2College of Pharmacy, Chung-Ang University, Seoul 06974, Korea. simss@cau.ac.kr
- KMID: 2443616
- DOI: http://doi.org/10.4196/kjpp.2019.23.1.55
Abstract
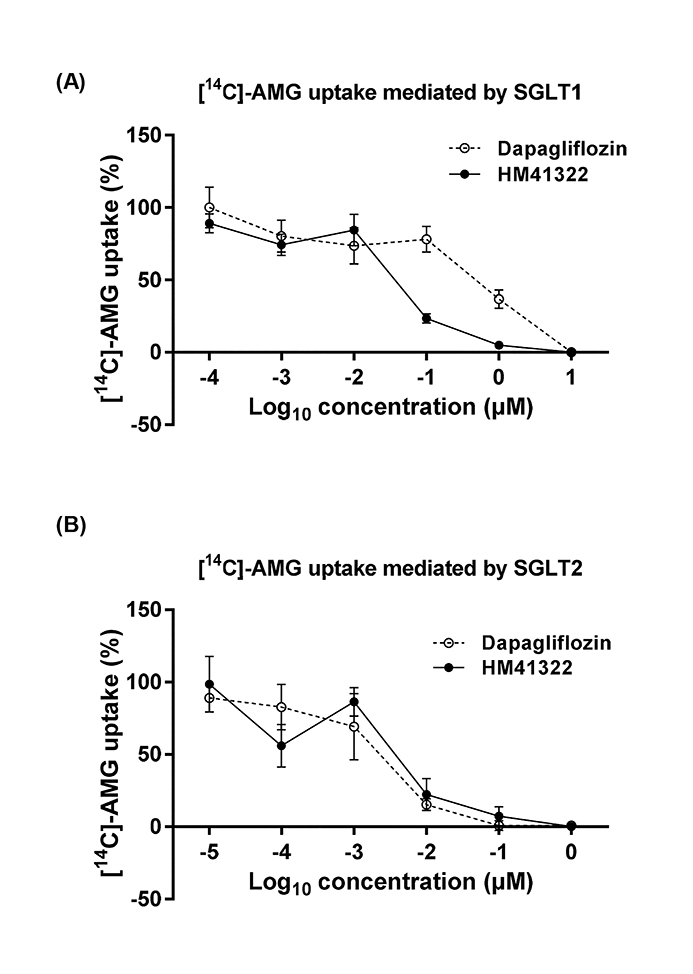
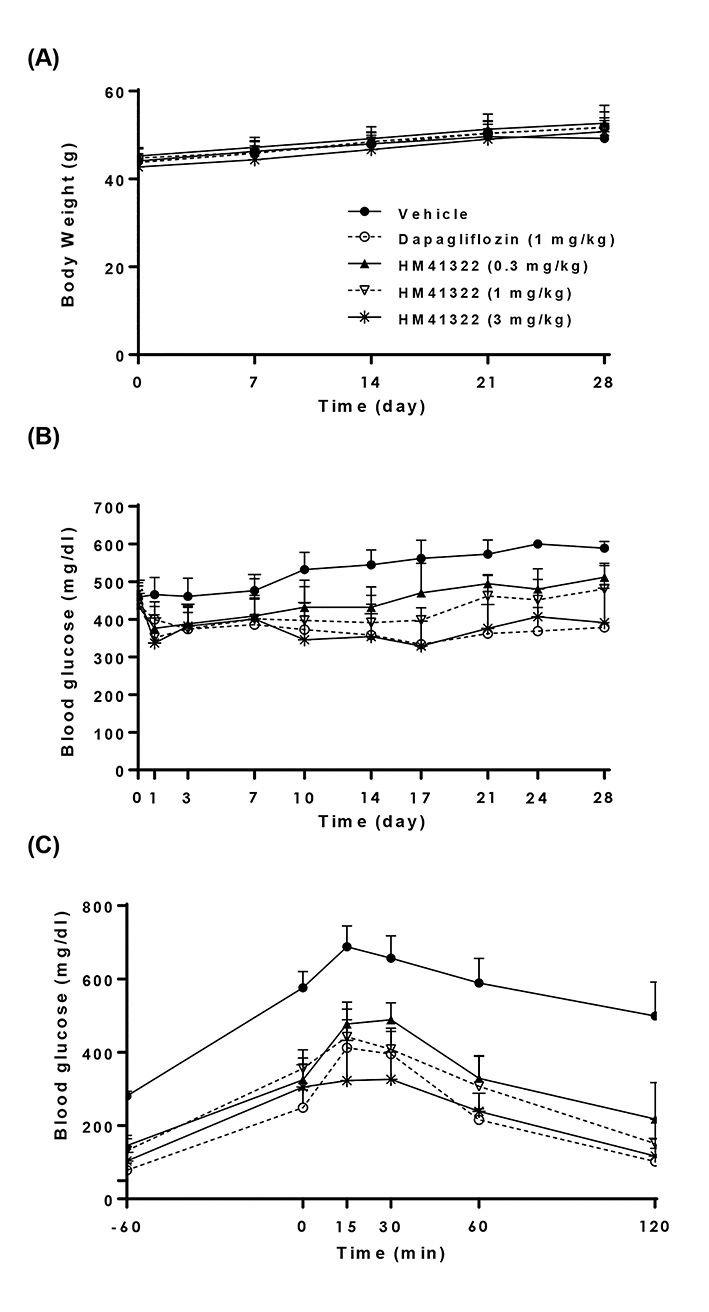
- HM41322 is a novel oral sodium-glucose cotransporter (SGLT) 1/2 dual inhibitor. In this study, the in vitro and in vivo pharmacokinetic and pharmacologic profiles of HM41322 were compared to those of dapagliflozin. HM41322 showed a 10-fold selectivity for SGLT2 over SGLT1. HM41322 showed an inhibitory effect on SGLT2 similar to dapagliflozin, but showed a more potent inhibitory effect on SGLT1 than dapagliflozin. The maximum plasma HM41322 level after single oral doses at 0.1, 1, and 3 mg/kg were 142, 439, and 1830 ng/ml, respectively, and the T(1/2) was 3.1 h. HM41322 was rapidly absorbed and reached the circulation within 15 min. HM41322 maximized urinary glucose excretion by inhibiting both SGLT1 and SGLT2 in the kidney. HM41322 3 mg/kg caused the maximum urinary glucose excretion in normoglycemic mice (19.32±1.16 mg/g) at 24 h. In normal and diabetic mice, HM41322 significantly reduced glucose excursion. Four-week administration of HM41322 in db/db mice reduced HbA1c in a dose dependent manner. Taken together, HM41322 showed a favorable preclinical profile of postprandial glucose control through dual inhibitory activities against SGLT1 and SGLT2.
Figure
Reference
-
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010; 33:Suppl 1. S62–S69.2. Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005; 11:2279–2299.
Article3. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005; 54:1–7.
Article4. Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016; 164:740–751.5. Cefalu WT. Pharmacotherapy for the treatment of patients with type 2 diabetes mellitus: rationale and specific agents. Clin Pharmacol Ther. 2007; 81:636–649.
Article6. Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994; 93:397–404.7. Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011; 22:104–112.
Article8. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011; 91:733–794.
Article9. Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, Sabolic I. Revised immunolocalization of the Na+-D-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol. 2008; 295:C475–C489.10. Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol. 2014; 306:F188–F193.
Article11. Wilding JP. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism. 2014; 63:1228–1237.
Article12. Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium-glucose cotransporter inhibitors: effects on renal and intestinal glucose transport: from bench to bedside. Diabetes Care. 2015; 38:2344–2353.13. Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, et al. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012; 61:187–196.14. Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes. 2013; 62:3324–3328.
Article15. Powell DR, DaCosta CM, Smith M, Doree D, Harris A, Buhring L, Heydorn W, Nouraldeen A, Xiong W, Yalamanchili P, Mseeh F, Wilson A, Shadoan M, Zambrowicz B, Ding ZM. Effect of LX4211 on glucose homeostasis and body composition in preclinical modelsr. J Pharmacol Exp Ther. 2014; 350:232–242.16. Zambrowicz B, Freiman J, Brown PM, Frazier KS, Turnage A, Bronner J, Ruff D, Shadoan M, Banks P, Mseeh F, Rawlins DB, Goodwin NC, Mabon R, Harrison BA, Wilson A, Sands A, Powell DR. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharmacol Ther. 2012; 92:158–169.
Article17. Powell DR, Smith MG, Doree DD, Harris AL, Xiong WW, Mseeh F, Wilson A, Gopinathan S, Diaz D, Goodwin NC, Harrison B, Strobel E, Rawlins DB, Carson K, Zambrowicz B, Ding ZM. LP-925219 maximizes urinary glucose excretion in mice by inhibiting both renal SGLT1 and SGLT2. Pharmacol Res Perspect. 2015; 3:e00129.
Article18. Powell DR, DaCosta CM, Gay J, Ding ZM, Smith M, Greer J, Doree D, Jeter-Jones S, Mseeh F, Rodriguez LA, Harris A, Buhring L, Platt KA, Vogel P, Brommage R, Shadoan MK, Sands AT, Zambrowicz B. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol Endocrinol Metab. 2013; 304:E117–E130.
Article19. Powell DR, Smith M, Greer J, Harris A, Zhao S, DaCosta C, Mseeh F, Shadoan MK, Sands A, Zambrowicz B, Ding ZM. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J Pharmacol Exp Ther. 2013; 345:250–259.
Article20. Kohler S, Zeller C, Iliev H, Kaspers S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. Adv Ther. 2017; 34:1707–1726.
Article21. Karagiannis T, Bekiari E, Tsapas A. Canagliflozin in the treatment of type 2 diabetes: an evidence-based review of its place in therapy. Core Evid. 2017; 12:1–10.
Article22. Avogaro A, Giaccari A, Fioretto P, Genovese S, Purrello F, Giorgino F, Del Prato S. A consensus statement for the clinical use of the renal sodium-glucose co-transporter-2 inhibitor dapagliflozin in patients with type 2 diabetes mellitus. Expert Rev Clin Pharmacol. 2017; 10:763–772.
Article23. Liang Y, Arakawa K, Ueta K, Matsushita Y, Kuriyama C, Martin T, Du F, Liu Y, Xu J, Conway B, Conway J, Polidori D, Ways K, Demarest K. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One. 2012; 7:e30555.
Article24. Riser Taylor S, Harris KB. The clinical efficacy and safety of sodium glucose cotransporter-2 inhibitors in adults with type 2 diabetes mellitus. Pharmacotherapy. 2013; 33:984–999.
Article25. Liu JJ, Lee T, DeFronzo RA. Why Do SGLT2 inhibitors inhibit only 30-50% of renal glucose reabsorption in humans? Diabetes. 2012; 61:2199–2204.
Article26. Shibazaki T, Tomae M, Ishikawa-Takemura Y, Fushimi N, Itoh F, Yamada M, Isaji M. KGA-2727, a novel selective inhibitor of a high-affinity sodium glucose cotransporter (SGLT1), exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther. 2012; 342:288–296.
Article27. Ikumi Y, Kida T, Sakuma S, Yamashita S, Akashi M. Polymer-phloridzin conjugates as an anti-diabetic drug that inhibits glucose absorption through the Na+/glucose cotransporter (SGLT1) in the small intestine. J Control Release. 2008; 125:42–49.28. Lapuerta P, Zambrowicz B, Strumph P, Sands A. Development of sotagliflozin, a dual sodium-dependent glucose transporter 1/2 inhibitor. Diab Vasc Dis Res. 2015; 12:101–110.
Article29. Yoder SM, Yang Q, Kindel TL, Tso P. Differential responses of the incretin hormones GIP and GLP-1 to increasing doses of dietary carbohydrate but not dietary protein in lean rats. Am J Physiol Gastrointest Liver Physiol. 2010; 299:G476–G485.
Article30. Vilsbøll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept. 2003; 114:115–121.
Article31. Takebayashi K, Inukai T. Effect of sodium glucose cotransporter 2 inhibitors with low SGLT2/SGLT1 selectivity on circulating glucagon-like peptide 1 levels in type 2 diabetes mellitus. J Clin Med Res. 2017; 9:745–753.
Article32. Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014; 124:499–508.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sodium-Glucose Cotransporter 2 Inhibitors for People with Type 1 Diabetes
- Evaluation of the anti-Toxoplasma gondii Activity of Hederagenin in vitro and in vivo
- Eribulin for Advanced Breast Cancer: A Drug Evaluation
- Dual Inhibitors Against Topoisomerases and Histone Deacetylases
- Effect of ROCK Inhibitor on the Expansion and Wound Healing of Human Corneal Endothelial Cell