Korean J Ophthalmol.
2019 Apr;33(2):122-130. 10.3341/kjo.2018.0037.
Switching to Aflibercept in Diabetic Macular Edema after Unsatisfactory Response to Other Anti-vascular Endothelial Growth Factor Drugs
- Affiliations
-
- 1Department of Ophthalmology, Assiut University, Assiut, Egypt. dr_ziadeldaly@aun.edu.eg
- 2Department of Ophthalmology, South Valley University, Qena, Egypt.
- KMID: 2442616
- DOI: http://doi.org/10.3341/kjo.2018.0037
Abstract
- PURPOSE
To evaluate the efficacy of switching to aflibercept in diabetic macular edema (DME) with suboptimal response to previous anti-vascular endothelial growth factor (anti-VEGF) injections.
METHODS
A prospective interventional case series study recruited patients from a single center diagnosed with DME with suboptimal response to anti-VEGF injections. Three consecutive monthly injections of aflibercept were performed. The primary outcome measure was mean change in visual acuity after switching to aflibercept.
RESULTS
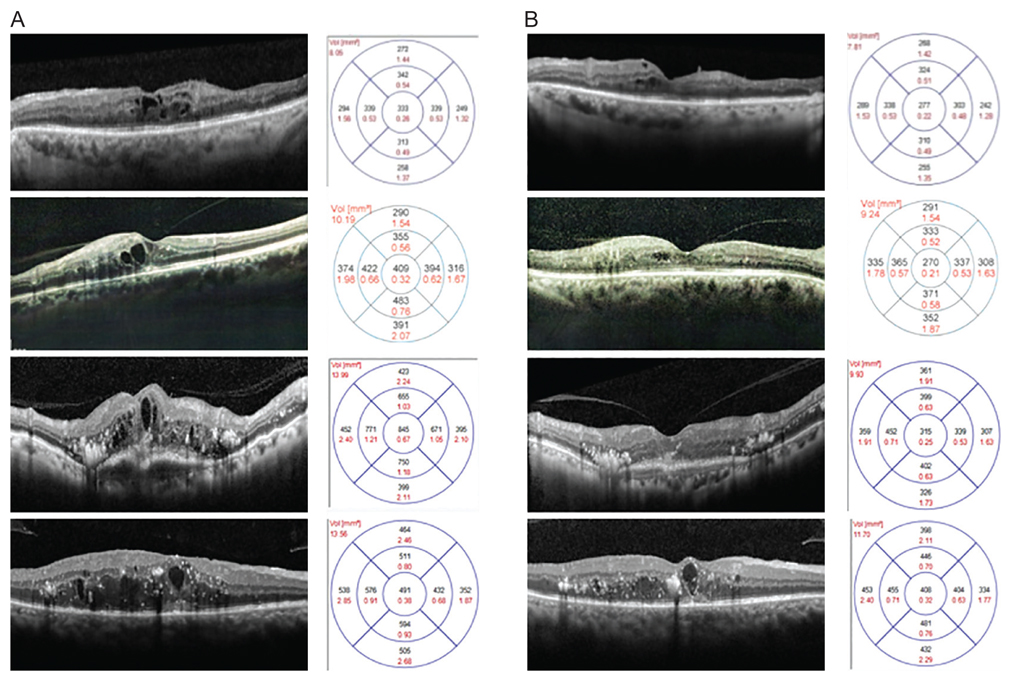
Forty-two patients (42 eyes) were included. Baseline logarithm of the minimum angle of resolution (logMAR) visual acuity was 0.87 ± 0.23 and improved significantly to 0.62 ± 0.29, 0.56 ± 0.34, and 0.46 ± 0.35 at 1, 2, and 3 months, respectively, after the first injection. Mean baseline retinal thickness was 451.57 ± 107.09 µm and decreased significantly at 1, 2, and 3 months after switching to aflibercept (346.52 ± 79.03, 328.24 ± 81.98, and 313.71 ± 85.79 µm, respectively). Both visual improvement and mean change in retinal thickness were significant in patients with pre-aflibercept best-corrected visual acuity less than 1.0 logMAR but were not significant in patients with best-corrected visual acuity more than 1.0 logMAR.
CONCLUSIONS
Switching to aflibercept in DME patients with an unsatisfactory response to previous anti-VEGF injections provided acceptable short-term visual and retinal architectural improvement.
Keyword
MeSH Terms
Figure
Reference
-
1. Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016; 44:260–277.
Article2. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35:556–564.3. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985; 103:1796–1806.4. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized t r ials: R ISE and R IDE. Ophthalmolog y. 2012; 119:789–801.5. Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012; 130:972–979.
Article6. Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015; 122:2044–2052.7. Do DV, Nguyen QD, Boyer D, et al. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012; 119:1658–1665.
Article8. Diabetic Retinopathy Clinical Research Network. Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015; 372:1193–1203.
Article9. Rahimy E, Shahlaee A, Khan MA, et al. Conversion to aflibercept after prior anti-VEGF therapy for persistent diabetic macular edema. Am J Ophthalmol. 2016; 164:118–127.
Article10. Bahrami B, Hong T, Zhu M, et al. Switching therapy from bevacizumab to aflibercept for the management of persistent diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017; 255:1133–1140.
Article11. Chen YY, Chang PY, Wang JK. Intravitreal aflibercept for patients with diabetic macular edema refractory to bevacizumab or ranibizumab: analysis of response to aflibercept. Asia Pac J Ophthalmol (Phila). 2017; 6:250–255.
Article12. Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012; 15:171–185.
Article13. Moradi A, Sepah YJ, Sadiq MA, et al. Vascular endothelial growth factor trap-eye (Aflibercept) for the management of diabetic macular edema. World J Diabetes. 2013; 4:303–309.
Article14. Stewart MW, Rosenfeld PJ, Penha FM, et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina. 2012; 32:434–457.
Article15. Pacella F, Romano MR, Turchetti P, et al. An eighteen-month follow-up study on the effects of Intravitreal Dexamethasone Implant in diabetic macular edema refractory to anti-VEGF therapy. Int J Ophthalmol. 2016; 9:1427–1432.
Article16. Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology. 2008; 115:2199–2205.
Article17. Gokce G, Durukan AH, Koylu MT, Kucukevcilioglu M. Efficacy of aflibercept on exudative age-related macular degeneration in patients exhibiting complete ranibizumab resistance and tachy phylaxis. Arq Bras Of talmol. 2016; 79:384–389.
Article18. Arjamaa O, Minn H. Resistance, not tachyphylaxis or tolerance. Br J Ophthalmol. 2012; 96:1153–1154.
Article19. Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol. 2012; 96:1–2.
Article20. Forooghian F, Chew EY, Meyerle CB, et al. Investigation of the role of neutralizing antibodies against bevacizumab as mediators of tachyphylaxis. Acta Ophthalmol. 2011; 89:e206–e207.
Article21. Praidou A, Androudi S, Brazitikos P, et al. Angiogenic growth factors and their inhibitors in diabetic retinopathy. Curr Diabetes Rev. 2010; 6:304–312.
Article22. Krizova L, Kalousova M, Kubena AA, et al. Correlation of vitreous vascular endothelial growth factor and uric acid concentration using optical coherence tomography in diabetic macular edema. J Ophthalmol. 2015; 2015:478509.
Article23. Ferris FL 3rd, Maguire MG, Glassman AR, et al. Evaluating effects of switching anti-vascular endothelial growth factor drugs for age-related macular degeneration and diabetic macular edema. JAMA Ophthalmol. 2016; 12. 22. DOI: 10.1001/jamaophthalmol.2016.4820.
Article24. Khan Z, Kuriakose RK, Khan M, et al. Efficacy of the intravitreal sustained-release dexamethasone implant for diabetic macular edema refractory to anti-vascular endothelial growth factor therapy: meta-analysis and clinical implications. Ophthalmic Surg Lasers Imaging Retina. 2017; 48:160–166.
Article25. Maturi RK, Bleau L, Saunders J, et al. A 12-month, single-masked, randomized controlled study of eyes with persistent diabetic macular edema after multiple anti-vegf injections to assess the efficacy of the dexamethasone-delayed delivery system as an adjunct to bevacizumab compared with continued bevacizumab monotherapy. Retina. 2015; 35:1604–1614.
Article26. Ghassemi F, Bazvand F, Roohipoor R, et al. Outcomes of vitrectomy, membranectomy and internal limiting membrane peeling in patients with refractory diabetic macular edema and non-tractional epiretinal membrane. J Curr Ophthalmol. 2016; 28:199–205.
Article27. Pieramici DJ, Wang PW, Ding B, Gune S. Visual and anatomic outcomes in patients with diabetic macular edema with limited initial anatomic response to ranibizumab in RIDE and RISE. Ophthalmology. 2016; 123:1345–1350.
Article28. Prabhu M, Kakhandaki A, Chandra KR, Dinesh MB. A hospital based study regarding awareness of association between glycosylated haemoglobin and severity of diabetic retinopathy in type 2 diabetic individuals. J Clin Diagn Res. 2016; 10:NC01–NC04.
Article29. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016; 123:1351–1359.30. Dugel PU, Hillenkamp J, Sivaprasad S, et al. Baseline visual acuity strongly predicts visual acuity gain in patients with diabetic macular edema following anti-vascular endothelial growth factor treatment across trials. Clin Ophthalmol. 2016; 10:1103–1110.
Article31. Wells JA, Glassman AR, Jampol LM, et al. Association of baseline visual acuity and retinal thickness with 1-year efficacy of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema. JAMA Ophthalmol. 2016; 134:127–134.
Article32. Eliwa TF, Hussein MA, Zaki MA, Raslan OA. Outer retinal layer thickness as good visual predictor in patients with diabetic macular edema. Retina. 2018; 38:805–811.
Article33. Sun JK, Radwan SH, Soliman AZ, et al. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes. 2015; 64:2560–2570.
Article34. Lee J, Moon BG, Cho AR, Yoon YH. Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology. 2016; 123:2368–2375.
Article35. Wong Y, Steel DH, Habib MS, et al. Vitreoretinal interface abnormalities in patients treatedwith ranibizumab for diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol. 2017; 255:733–742.
Article36. Sadiq MA, Soliman MK, Sarwar S, et al. Effect of vitreomacular adhesion on treatment outcomes in the ranibizumab for edema of the macula in diabetes (READ-3) study. Ophthalmology. 2016; 123:324–329.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Multimodal Approach to Diabetic Macular Edema
- Intravitreal injection of anti-vascular endothelial growth factor for patients with various retinal diseases
- Aflibercept Treatment for Neovascular Age-related Macular Degeneration and Polypoidal Choroidal Vasculopathy Refractory to Anti-vascular Endothelial Growth Factor
- Limited Treatment Response during Follow-up after Switching to Aflibercept in Neovascular Age-related Macular Degeneration
- Meta-analysis of Intravitreal Injection of Anti-vascular Endothelial Growth Factors for Diabetic Macular Edema