Lab Med Online.
2019 Apr;9(2):94-98. 10.3343/lmo.2019.9.2.94.
A Case of Urinary Tract Infection Caused by the Emerging Uropathogen Actinotignum schaalii
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. pmhhj77@gmail.com
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Green Cross Genome, Yongin, Korea.
- KMID: 2441830
- DOI: http://doi.org/10.3343/lmo.2019.9.2.94
Abstract
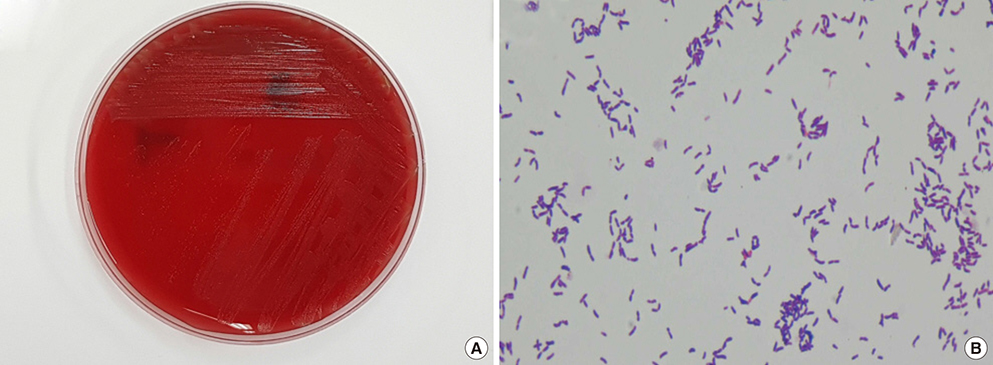
- Actinotignum schaalii is an emerging uropathogen; however, routine culture protocols and usual phenotypic methods do not allow for easy detection and identification. Herein, we report the first Korean case of urinary tract infection caused by A. schaalii in a 79-year-old patient with prostate cancer. A gram-positive rod bacterium was isolated from the patient's urine after 2 days of culture and identified as A. schaalii using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry and DNA target sequencing.
MeSH Terms
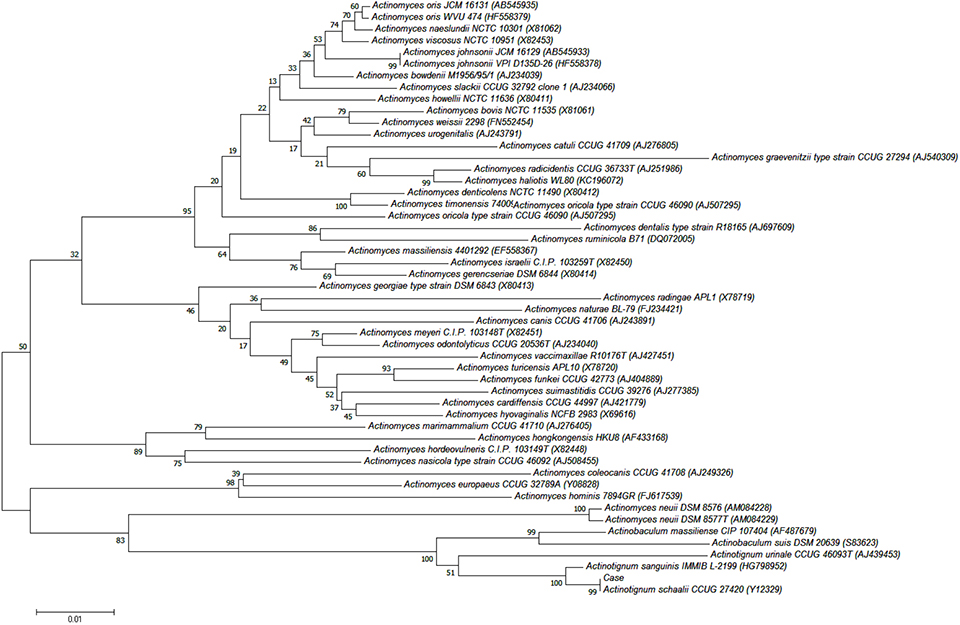
Figure
Reference
-
1. Lotte R, Lotte L, Ruimy R. Actinotignum schaalii (formerly Actinobaculum schaalii): a newly recognized pathogen-review of the literature. Clin Microbiol Infect. 2016; 22:28–36.
Article2. Tena D, Fernández C, Lago MR, Arias M, Medina MJ, Sáez-Nieto JA. Skin and soft-tissue infections caused by Actinobaculum schaalii: report of two cases and literature review. Anaerobe. 2014; 28:95–97.
Article3. Haller P, Bruderer T, Schaeren S, Laifer G, Frei R, Battegay M, et al. Vertebral osteomyelitis caused by Actinobaculum schaalii: a difficult-to-diagnose and potentially invasive uropathogen. Eur J Clin Microbiol Infect Dis. 2007; 26:667–670.
Article4. Hoenigl M, Leitner E, Valentin T, Zarfel G, Salzer HJ, Krause R, et al. Endocarditis caused by Actinobaculum schaalii, Austria. Emerg Infect Dis. 2010; 16:1171–1173.5. Clinical and Laboratory Standards Institute. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing; approved guideline. CLSI document MM18-A. Wayne, PA: Clinical and Laboratory Standards Institute;2008.6. Yassin AF, Spröer C, Pukall R, Sylvester M, Siering C, Schumann P. Dissection of the genus Actinobaculum: Reclassification of Actinobaculum schaalii Lawson et al. 1997 and Actinobaculum urinale Hall et al. 2003 as Actinotignum schaalii gen. nov., comb. nov. and Actinotignum urinale comb. nov., description of Actinotignum sanguinis sp. nov. and emended descriptions of the genus Actinobaculum and Actinobaculum suis; and re-examination of the culture deposited as Actinobaculum massiliense CCUG 47753T (=DSM 19118T), revealing that it does not represent a strain of this species. Int J Syst Evol Microbiol. 2015; 65:615–624.
Article7. Lawson PA, Falsen E, Akervall E, Vandamme P, Collins MD. Characterization of some Actinomyces-like isolates from human clinical specimens: reclassification of Actinomyces suis (Soltys and Spratling) as Actinobaculum suis comb. nov. and description of Actinobaculum schaalii sp. nov. Int J Syst Bacteriol. 1997; 47:899–903.
Article8. Fendukly F, Osterman B. Isolation of Actinobaculum schaalii and Actinobaculum urinale from a patient with chronic renal failure. J Clin Microbiol. 2005; 43:3567–3569.
Article9. Hall V, Collins MD, Hutson RA, Falsen E, Inganäs E, Duerden BI. Actinobaculum urinale sp. nov., from human urine. Int J Syst Evol Microbiol. 2003; 53:679–682.10. Cattoir V. Actinobaculum schaalii: review of an emerging uropathogen. J Infect. 2012; 64:260–267.11. Stevens RP, Taylor PC. Actinotignum (formerly Actinobaculum) schaalii: a review of MALDI-TOF for identification of clinical isolates, and a proposed method for presumptive phenotypic identification. Pathology. 2016; 48:367–371.
Article12. Nielsen HL, Søby KM, Christensen JJ, Prag J. Actinobaculum schaalii: a common cause of urinary tract infection in the elderly population. Bacteriological and clinical characteristics. Scand J Infect Dis. 2010; 42:43–47.
Article13. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002; 113:S1A. 5S–13S.
Article14. McKew G, Watson B, Chan R, van Hal SJ. “Probable contaminants” no more: rapid identification of Gram-positive rods leads to improved clinical care. J Clin Microbiol. 2013; 51:1641.
Article15. Tuuminen T, Suomala P, Harju I. Actinobaculum schaalii: identification with MALDI-TOF. New Microbes New Infect. 2014; 2:38–41.16. Cattoir V, Varca A, Greub G, Prod'hom G, Legrand P, Lienhard R. In vitro susceptibility of Actinobaculum schaalii to 12 antimicrobial agents and molecular analysis of fluoroquinolone resistance. J Antimicrob Chemother. 2010; 65:2514–2517.
Article17. Reinhard M, Prag J, Kemp M, Andresen K, Klemmensen B, Højlyng N, et al. Ten cases of Actinobaculum schaalii infection: clinical relevance, bacterial identification, and antibiotic susceptibility. J Clin Microbiol. 2005; 43:5305–5308.
Article18. Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, Fitzgerald M, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012; 50:1376–1383.
Article19. Olsen AB, Andersen PK, Bank S, Søby KM, Lund L, Prag J. Actinobaculum schaalii, a commensal of the urogenital area. BJU Int. 2013; 112:394–397.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Investigation of Causative Organisms and Resistance to Antibiotics in Urinary Tract Infection
- A Case of Infantile Fungal Urinary Tract Infection
- Screening of urinary tract infection in high-risk neonates
- Urinary Tract Infection Caused by Escherichia coli
- Clinical Evaluation of Furadantin C for Urinary Tract Infection