J Gynecol Oncol.
2019 May;30(3):e28. 10.3802/jgo.2019.30.e28.
A multi-institutional analysis of sequential versus ‘sandwich’ adjuvant chemotherapy and radiotherapy for stage IIIC endometrial carcinoma
- Affiliations
-
- 1Department of Radiation Oncology, Baskent University Faculty of Medicine, Adana Dr. Turgut Noyan Research and Treatment Center, Adana, Turkey. hcemonal@hotmail.com
- 2Department of Radiation Oncology, Hacettepe University Faculty of Medicine, Ankara, Turkey.
- 3Department of Radiation Oncology, Selcuk University Faculty of Medicine, Konya, Turkey.
- 4Department of Radiation Oncology, Ankara University Faculty of Medicine, Ankara, Turkey.
- KMID: 2440512
- DOI: http://doi.org/10.3802/jgo.2019.30.e28
Abstract
OBJECTIVE
To analyze the outcomes of sequential or sandwich chemotherapy (ChT) and radiotherapy (RT) in patients with node-positive endometrial cancer (EC).
METHODS
Data from 4 centers were collected retrospectively for 179 patients with stage IIIC EC treated with postoperative RT and ChT (paclitaxel and carboplatin). Patients were either treated with 6 cycles of ChT followed by RT (sequential arm; 96 patients) or with 3 cycles of ChT, RT, and an additional 3 cycles of ChT (sandwich arm; 83 patients). Prognostic factors affecting overall survival (OS) and progression-free survival (PFS) were analyzed.
RESULTS
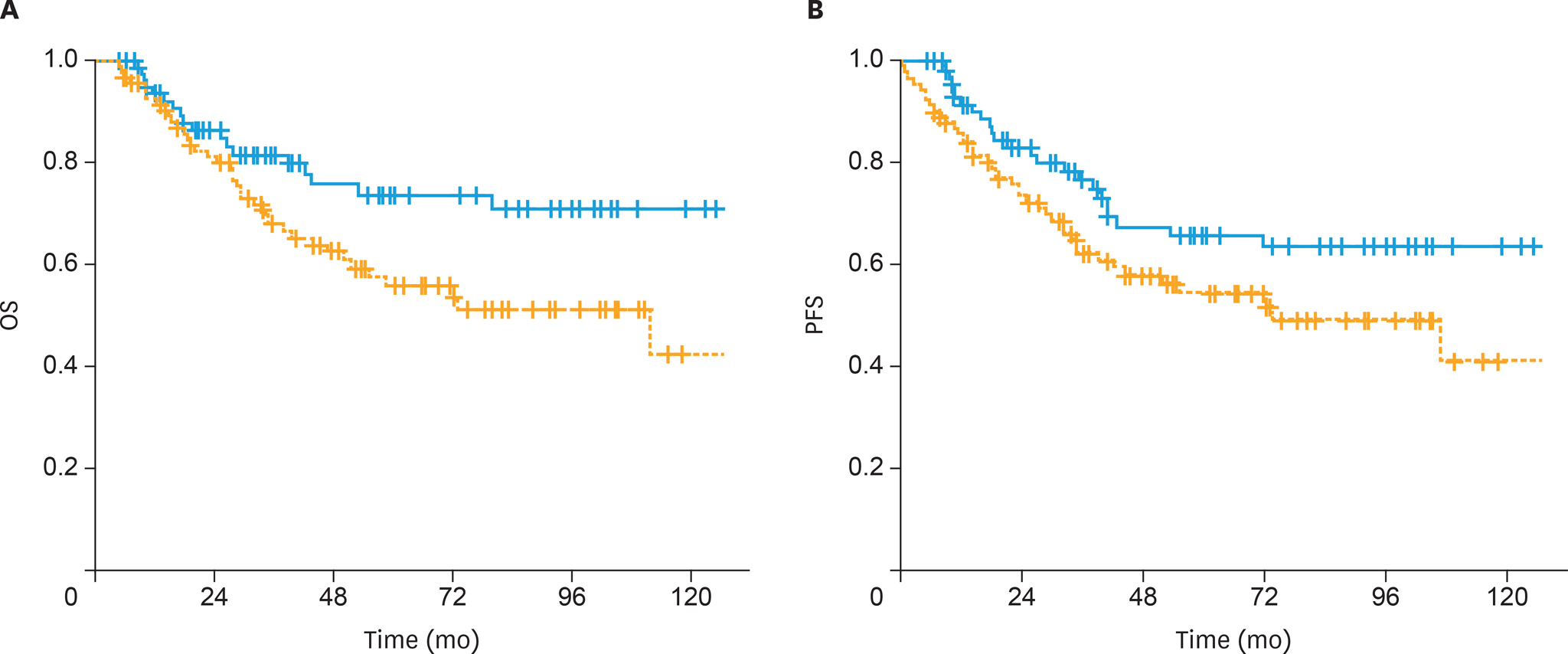
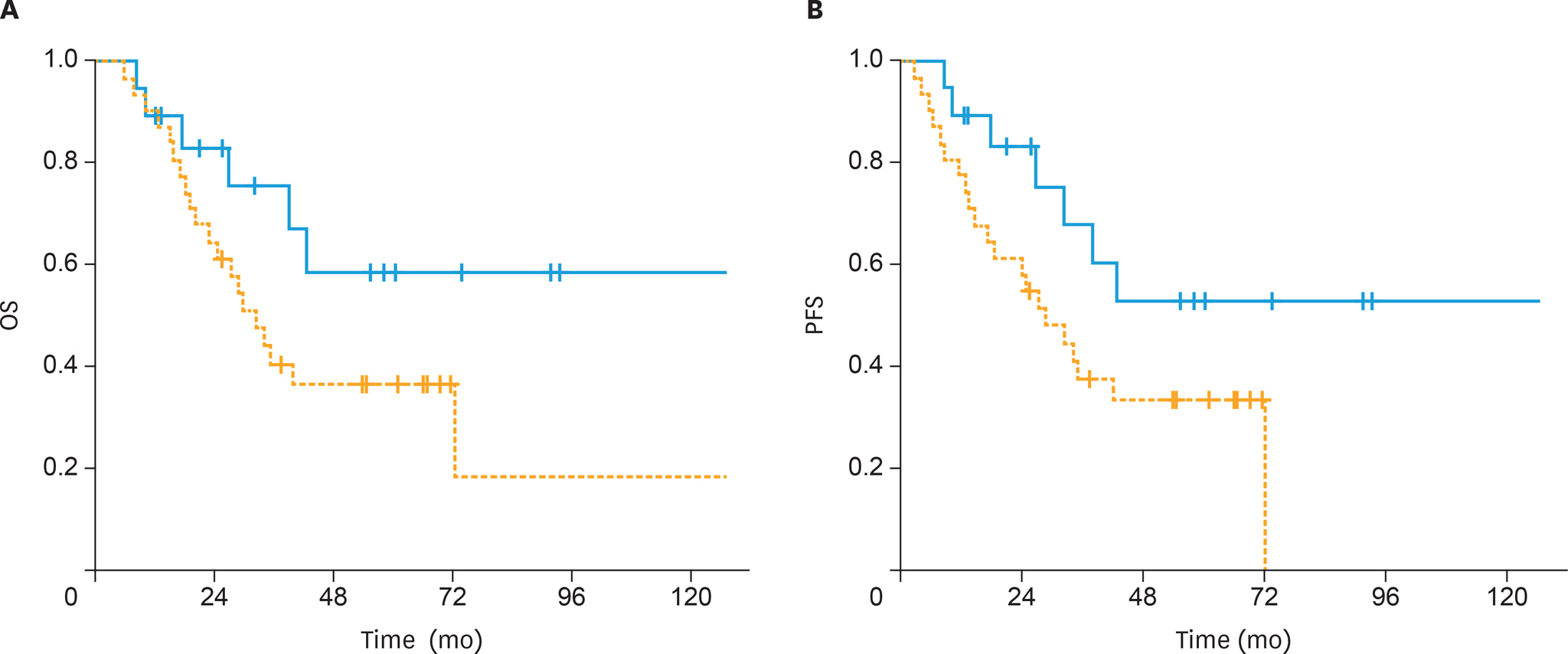
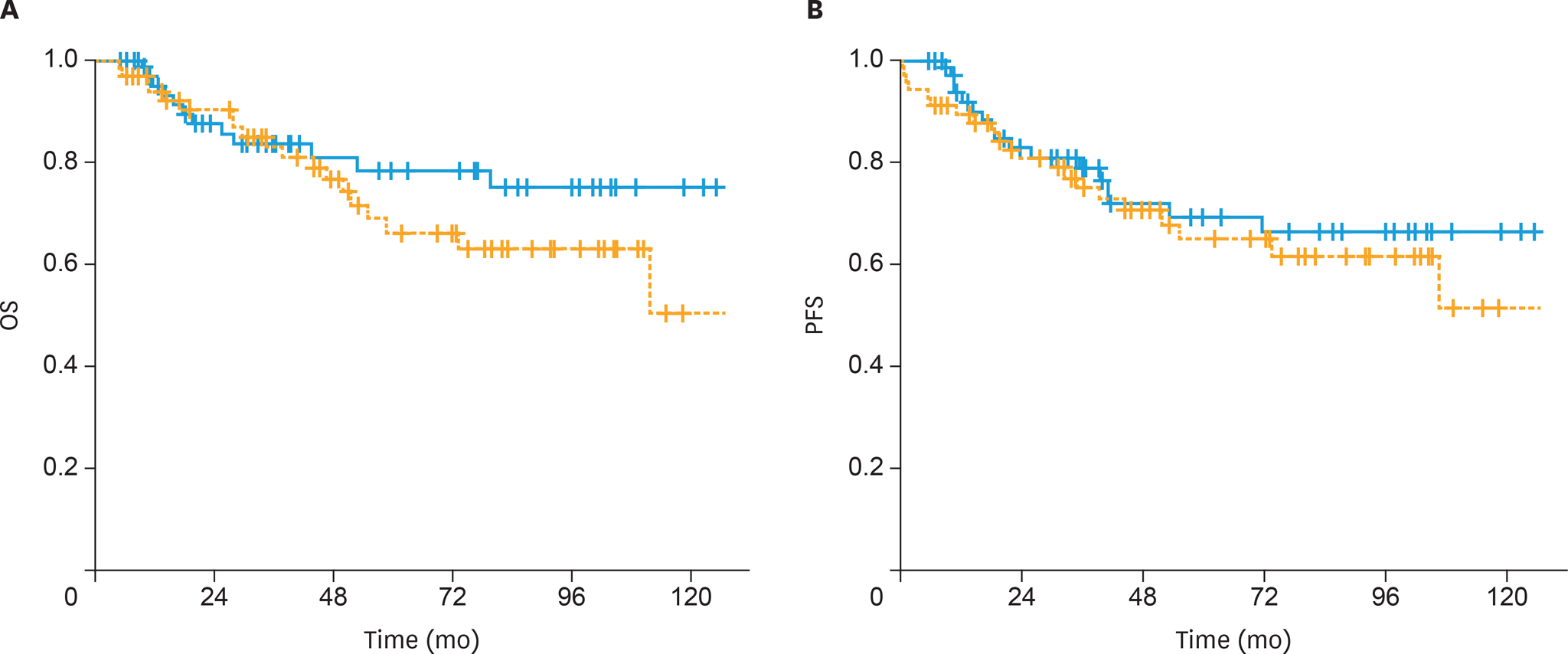
The 5-year OS and PFS rates were 64% and 59%, respectively, with a median follow-up of 41 months (range, 5-167 months). The 5-year OS rates were significantly higher in the sandwich than sequential arms (74% vs. 56%; p=0.03) and the difference for 5-year PFS rates was nearly significant (65% vs. 54%; p=0.05). In univariate analysis, treatment strategy, age, International Federation of Gynecology and Obstetrics (FIGO) stage, pathology, rate of myometrial invasion, and grade were prognostic factors for OS and PFS. In multivariate analysis, non-endometrioid histology, advanced FIGO stage, and adjuvant sequential ChT and RT were negative predictors for OS, whereas only non-endometrioid histology was a prognostic factor for PFS.
CONCLUSION
Postoperative adjuvant ChT and RT for stage IIIC EC patients, either given sequentially or sandwiched, offers excellent clinical efficacy and acceptably low toxicity. Our data support the superiority of the sandwich regimen compared to the sequential strategy in stage IIIC EC patients for OS.
MeSH Terms
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article2. Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017; 109:djx030.
Article3. Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC study group. Post operative radiation therapy in endometrial carcinoma. Lancet. 2000; 355:1404–11.4. Papanikolaou A, Kalogiannidis I, Goutzioulis M, Misailidou D, Makedos A, Vergote I, et al. Pelvic lymphadenectomy as alternative to postoperative radiotherapy in high risk early stage endometrial cancer. Arch Gynecol Obstet. 2006; 274:91–6.
Article5. Lewin SN, Herzog TJ, Barrena Medel NI, Deutsch I, Burke WM, Sun X, et al. Comparative performance of the 2009 international federation of gynecology and obstetrics' staging system for uterine corpus cancer. Obstet Gynecol. 2010; 116:1141–9.
Article6. McMeekin DS, Lashbrook D, Gold M, Johnson G, Walker JL, Mannel R. Analysis of FIGO stage IIIc endometrial cancer patients. Gynecol Oncol. 2001; 81:273–8.
Article7. Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol. 2006; 24:36–44.
Article8. Smith RS, Kapp DS, Chen Q, Teng NN. Treatment of high-risk uterine cancer with whole abdominopelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2000; 48:767–78.
Article9. Shaikh T, Churilla TM, Mantia-Smaldone GM, Chu C, Rubin SC, Anderson PR. The role of adjuvant radiation in lymph node positive endometrial adenocarcinoma. Gynecol Oncol. 2016; 141:434–9.
Article10. Mariani A, Dowdy SC, Cliby WA, Haddock MG, Keeney GL, Lesnick TG, et al. Efficacy of systematic lymphadenectomy and adjuvant radiotherapy in node-positive endometrial cancer patients. Gynecol Oncol. 2006; 101:200–8.
Article11. van Wijk FH, van der Burg ME, Burger CW, Vergote I, van Doorn HC. Management of surgical stage III and IV endometrioid endometrial carcinoma: an overview. Int J Gynecol Cancer. 2009; 19:431–46.
Article12. Boothe D, Orton A, Odei B, Stoddard G, Suneja G, Poppe MM, et al. Chemoradiation versus chemotherapy or radiation alone in stage III endometrial cancer: patterns of care and impact on overall survival. Gynecol Oncol. 2016; 141:421–7.
Article13. Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer–results from two randomised studies. Eur J Cancer. 2010; 46:2422–31.
Article14. Geller MA, Ivy J, Dusenbery KE, Ghebre R, Isaksson Vogel R, Argenta PA. A single institution experience using sequential multi-modality adjuvant chemotherapy and radiation in the “sandwich” method for high risk endometrial carcinoma. Gynecol Oncol. 2010; 118:19–23.
Article15. Secord AA, Havrilesky LJ, O'Malley DM, Bae-Jump V, Fleming ND, Broadwater G, et al. A multicenter evaluation of sequential multimodality therapy and clinical outcome for the treatment of advanced endometrial cancer. Gynecol Oncol. 2009; 114:442–7.
Article16. Lan C, Huang X, Cao X, Huang H, Feng Y, Huang Y, et al. Adjuvant docetaxel and carboplatin chemotherapy administered alone or with radiotherapy in a “sandwich” protocol in patients with advanced endometrial cancer: a single-institution experience. Expert Opin Pharmacother. 2013; 14:535–42.
Article17. Lu SM, Chang-Halpenny C, Hwang-Graziano J. Sequential versus “sandwich” sequencing of adjuvant chemoradiation for the treatment of stage III uterine endometrioid adenocarcinoma. Gynecol Oncol. 2015; 137:28–33.
Article18. Modh A, Ghanem AI, Burmeister C, Hanna RK, Elshaikh MA. What is the optimal adjuvant treatment sequence for node-positive endometrial cancer? Results of a national cancer database analysis. Int J Gynecol Cancer. 2018; 28:248–53.
Article19. Gao H, Zhang Z. Sequential chemotherapy and radiotherapy in the sandwich method for advanced endometrial cancer: a meta-analysis. Medicine (Baltimore). 2015; 94:e672.20. Fields AL, Einstein MH, Novetsky AP, Gebb J, Goldberg GL. Pilot phase II trial of radiation “sandwiched” between combination paclitaxel/platinum chemotherapy in patients with uterine papillary serous carcinoma (UPSC). Gynecol Oncol. 2008; 108:201–6.
Article21. Lupe K, Kwon J, D'Souza D, Gawlik C, Stitt L, Whiston F, et al. Adjuvant paclitaxel and carboplatin chemotherapy with involved field radiation in advanced endometrial cancer: a sequential approach. Int J Radiat Oncol Biol Phys. 2007; 67:110–6.
Article22. Geller MA, Ivy JJ, Ghebre R, Downs LS Jr, Judson PL, Carson LF, et al. A phase II trial of carboplatin and docetaxel followed by radiotherapy given in a “sandwich” method for stage III, IV, and recurrent endometrial cancer. Gynecol Oncol. 2011; 121:112–7.
Article23. Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016; 26:2–30.
Article24. Klopp AH, Jhingran A, Ramondetta L, Lu K, Gershenson DM, Eifel PJ. Node-positive adenocarcinoma of the endometrium: outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009; 115:6–11.
Article25. Gadducci A, Guerrieri ME, Cosio S, Fabrini MG, Laliscia C, Attianese D, et al. Rates, sites and times of recurrence and clinical outcome of endometrial cancer patients with histologically-positive nodes: an Italian two-center retrospective study. Anticancer Res. 2018; 38:1695–703.
Article26. Brown AP, Gaffney DK, Dodson MK, Soisson AP, Belnap TW, Alleman K, et al. Survival analysis of endometrial cancer patients with positive lymph nodes. Int J Gynecol Cancer. 2013; 23:861–8.
Article27. Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006; 95:266–71.
Article28. Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese gynecologic oncology group study. Gynecol Oncol. 2008; 108:226–33.
Article29. Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I–IV carcinosarcoma (CS) of the uterus. Gynecol Oncol. 2007; 107:177–85.
Article30. Johnson N, Bryant A, Miles T, Hogberg T, Cornes P. Adjuvant chemotherapy for endometrial cancer after hysterectomy. Cochrane Database Syst Rev. 2011; 10:CD003175.
Article31. Einstein MH, Frimer M, Kuo DY, Reimers LL, Mehta K, Mutyala S, et al. Phase II trial of adjuvant pelvic radiation “sandwiched” between combination paclitaxel and carboplatin in women with uterine papillary serous carcinoma. Gynecol Oncol. 2012; 124:21–5.
Article32. Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006; 103:155–9.
Article33. Wong AT, Rineer J, Lee YC, Schwartz D, Safdieh J, Weiner J, et al. Utilization of adjuvant therapies and their impact on survival for women with stage IIIC endometrial adenocarcinoma. Gynecol Oncol. 2016; 142:514–9.
Article34. Lee LJ, Viswanathan AN. Combined chemotherapy and radiation improves survival for node-positive endometrial cancer. Gynecol Oncol. 2012; 127:32–7.
Article35. Huddleston A, Zhen S, Qi L, Rash D, Leiserowitz G, Mayadev J. The impact of a vaginal brachytherapy boost to pelvic radiation in stage III endometrial cancer. J Contemp Brachytherapy. 2015; 7:122–7.
Article36. Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Chan J, et al. Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw. 2014; 12:248–80.
Article37. Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol. 2004; 22:2159–66.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Recurrent Endometrial Carcinoma at the Vagina
- Adjuvant sequential chemo and radiotherapy improves the oncological outcome in high risk endometrial cancer
- Adjuvant carboplatin and paclitaxel with “sandwich” method radiotherapy for stage III or IV endometrial cancer: long-term follow-up at a singleinstitution
- Postoperative Radiotherapy Alone Versus Chemoradiotherapy in Stage I-II Endometrial Carcinoma: An Investigational and Propensity Score Matching Analysis
- Adjuvant therapy in high-risk early endometrial carcinoma: a retrospective analysis of 46 cases