J Adv Prosthodont.
2019 Feb;11(1):48-54. 10.4047/jap.2019.11.1.48.
All-ceramic versus titanium-based implant supported restorations: Preliminary 12-months results from a randomized controlled trial
- Affiliations
-
- 1Department of Prosthodontics, School of Dentistry, Goethe-University Frankfurt am Main, Germany. weigl@em.uni-frankfurt.de
- 2Department of Oral Surgery and Implantology, School of Dentistry, Goethe-University Frankfurt am Main, Germany.
- KMID: 2438931
- DOI: http://doi.org/10.4047/jap.2019.11.1.48
Abstract
- PURPOSE
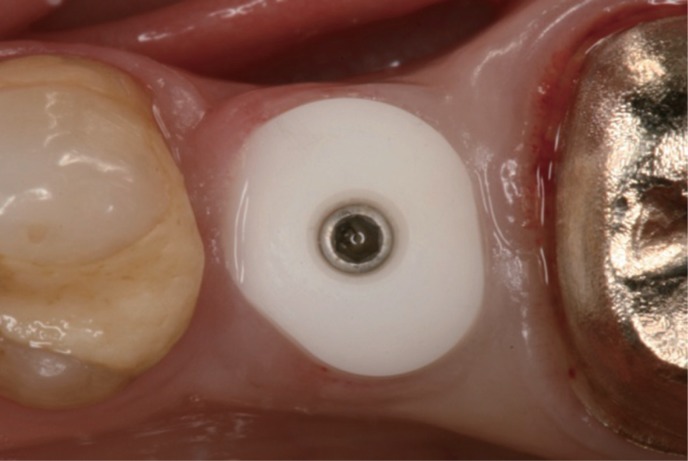
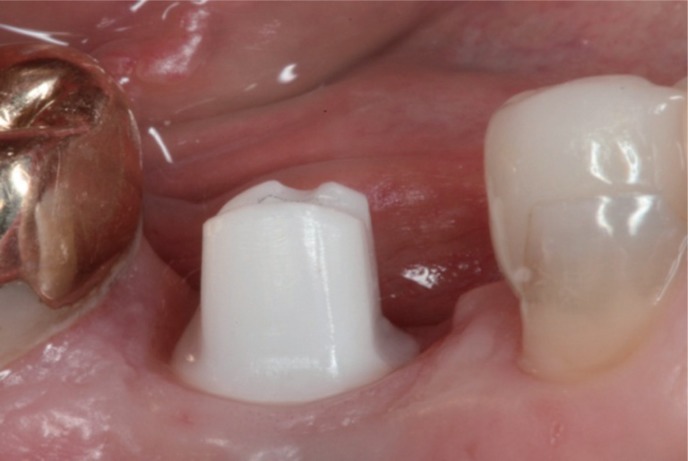
The aim of the present randomized controlled study was to compare prefabricated all-ceramic, anatomically shaped healing abutments followed by all-ceramic abutments and all-ceramic crowns and prefabricated standard-shaped (round-diameter) titanium healing abutments followed by final titanium abutments restored with porcelain-fused-to-metal (PFM) implant crowns in the premolar and molar regions.
MATERIALS AND METHODS
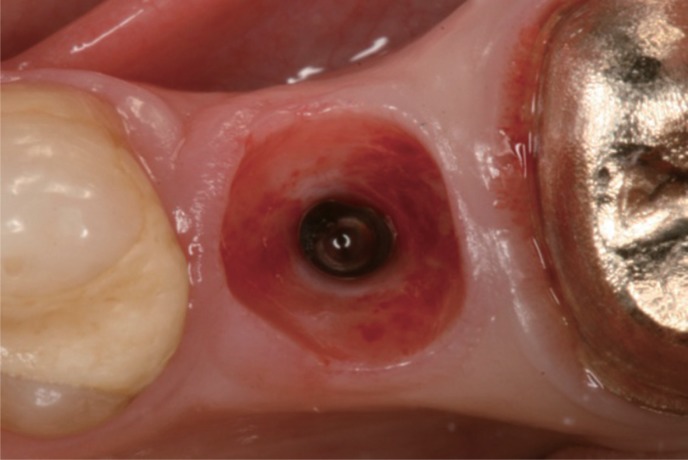
Forty-two patients received single implants restored either by all-ceramic restorations (test group, healing abutment, final abutment, and crown all made of zirconia) or conventional titanium-based restorations. Immediately after prosthetic incorporation and after 12 months of loading, implant survival, technical complications, bone loss, sulcus fluid flow rate (SFFR) as well as plaque index (PI) and implant stability (Periotest) were analyzed clinically and radiologically.
RESULTS
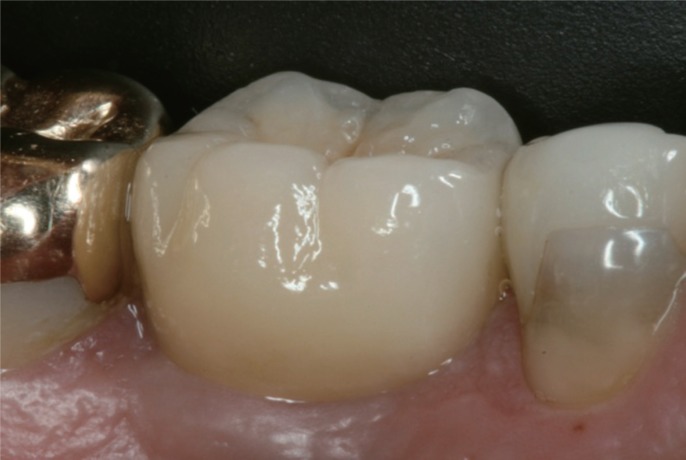
After 12 months of loading, an implant and prosthetic survival rate of 100% was observed. Minor prosthetic complications such as chipping of ceramic veneering occurred in both groups. No statistical significant differences were observed between both groups with only a minimum of bone loss, SFFR, and PI.
CONCLUSION
All-ceramic implant prostheses including a prefabricated anatomically shaped healing abutment achieved comparable results to titanium-based restorations in the posterior region. However, observational results indicate a benefit as shaping the peri-implant soft-tissue with successive provisional devices and subsequent compression of the soft tissue can be avoided.
Keyword
Figure
Reference
-
1. Linkevicius T, Puisys A, Linkeviciene L, Peciuliene V, Schlee M. Crestal bone stability around implants with horizontally matching connection after soft tissue thickening: A prospective clinical trial. Clin Implant Dent Relat Res. 2015; 17:497–508. PMID: 24103157.
Article2. Linkevicius T, Puisys A, Steigmann M, Vindasiute E, Linkeviciene L. Influence of vertical soft tissue thickness on crestal bone changes around implants with platform switching: A comparative clinical study. Clin Implant Dent Relat Res. 2015; 17:1228–1236. PMID: 24673875.
Article3. Bassetti RG, Stähli A, Bassetti MA, Sculean A. Soft tissue augmentation around osseointegrated and uncovered dental implants: a systematic review. Clin Oral Investig. 2017; 21:53–70.
Article4. Lewis MB, Klineberg I. Prosthodontic considerations designed to optimize outcomes for single-tooth implants. A review of the literature. Aust Dent J. 2011; 56:181–192. PMID: 21623811.
Article5. Furze D, Byrne A, Alam S, Wittneben JG. Esthetic outcome of implant supported crowns with and without peri-implant conditioning using provisional fixed prosthesis: A randomized controlled clinical trial. Clin Implant Dent Relat Res. 2016; 18:1153–1162. PMID: 26992007.
Article6. Esposito M, Bressan E, Grusovin MG, D'Avenia F, Neumann K, Sbricoli L, Luongo G. Do repeated changes of abutments have any influence on the stability of peri-implant tissues? One-year post-loading results from a multicentre randomised controlled trial. Eur J Oral Implantol. 2017; 10:57–72. PMID: 28327695.7. Alshhrani WM, Al Amri MD. Customized CAD-CAM healing abutment for delayed loaded implants. J Prosthet Dent. 2016; 116:176–179. PMID: 27038526.
Article8. Wittneben JG, Buser D, Belser UC, Brägger U. Peri-implant soft tissue conditioning with provisional restorations in the esthetic zone: the dynamic compression technique. Int J Periodontics Restorative Dent. 2013; 33:447–455. PMID: 23820704.
Article9. Lops D, Bressan E, Parpaiola A, Sbricoli L, Cecchinato D, Romeo E. Soft tissues stability of cad-cam and stock abutments in anterior regions: 2-year prospective multicentric cohort study. Clin Oral Implants Res. 2015; 26:1436–1442. PMID: 25196805.
Article10. Staubli N, Walter C, Schmidt JC, Weiger R, Zitzmann NU. Excess cement and the risk of peri-implant disease - a systematic review. Clin Oral Implants Res. 2017; 28:1278–1290. PMID: 27647536.
Article11. Linkevicius T, Vaitelis J. The effect of zirconia or titanium as abutment material on soft peri-implant tissues: a systematic review and meta-analysis. Clin Oral Implants Res. 2015; 26:139–147. PMID: 26073346.
Article12. Nothdurft FP, Nonhoff J, Pospiech PR. Pre-fabricated zirconium dioxide implant abutments for single-tooth replacement in the posterior region: success and failure after 3 years of function. Acta Odontol Scand. 2014; 72:392–400. PMID: 24304290.
Article13. Ekfeldt A, Fürst B, Carlsson GE. Zirconia abutments for single-tooth implant restorations: a retrospective and clinical follow-up study. Clin Oral Implants Res. 2011; 22:1308–1314. PMID: 21382085.
Article14. Quigley GA, Hein JW. Comparative cleansing efficiency of manual and power brushing. J Am Dent Assoc. 1962; 65:26–29. PMID: 14489483.
Article15. Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004; 75:292–296. PMID: 15068118.
Article16. Welander M, Abrahamsson I, Berglundh T. The mucosal barrier at implant abutments of different materials. Clin Oral Implants Res. 2008; 19:635–641. PMID: 18492075.
Article17. Pabst AM, Walter C, Bell A, Weyhrauch M, Schmidtmann I, Scheller H, Lehmann KM. Influence of CAD/CAM zirconia for implant-abutment manufacturing on gingival fibroblasts and oral keratinocytes. Clin Oral Investig. 2016; 20:1101–1108.
Article18. Sundh A, Molin M, Sjögren G. Fracture resistance of yttrium oxide partially-stabilized zirconia all-ceramic bridges after veneering and mechanical fatigue testing. Dent Mater. 2005; 21:476–482. PMID: 15826705.
Article19. Zembic A, Sailer I, Jung RE, Hämmerle CH. Randomized-controlled clinical trial of customized zirconia and titanium implant abutments for single-tooth implants in canine and posterior regions: 3-year results. Clin Oral Implants Res. 2009; 20:802–808. PMID: 19486077.
Article20. Sailer I, Zembic A, Jung RE, Siegenthaler D, Holderegger C, Hämmerle CH. Randomized controlled clinical trial of customized zirconia and titanium implant abutments for canine and posterior single-tooth implant reconstructions: preliminary results at 1 year of function. Clin Oral Implants Res. 2009; 20:219–225. PMID: 19397632.
Article21. Brägger U, Karoussis I, Persson R, Pjetursson B, Salvi G, Lang N. Technical and biological complications/failures with single crowns and fixed partial dentures on implants: a 10-year prospective cohort study. Clin Oral Implants Res. 2005; 16:326–334. PMID: 15877753.
Article22. Jung RE, Pjetursson BE, Glauser R, Zembic A, Zwahlen M, Lang NP. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin Oral Implants Res. 2008; 19:119–130. PMID: 18067597.
Article23. Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004; 75:292–296. PMID: 15068118.
Article24. Rimondini L, Cerroni L, Carrassi A, Torricelli P. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants. 2002; 17:793–798. PMID: 12507238.25. Sanz-Martín I, Sanz-Sánchez I, Carrillo de, Figuero E, Sanz M. Effects of modified abutment characteristics on peri-implant soft tissue health: A systematic review and meta-analysis. Clin Oral Implants Res. 2018; 29:118–129. PMID: 29072346.
Article26. Holländer J, Lorenz J, Stübinger S, Hölscher W, Heidemann D, Ghanaati S, Sader R. Zirconia dental implants: Investigation of clinical parameters, patient satisfaction, and microbial contamination. Int J Oral Maxillofac Implants. 2016; 31:855–864. PMID: 27447153.
Article27. Bösch A, Jung RE, Sailer I, Goran B, Hämmerle CH, Thoma DS. Single-tooth replacement using dental implants supporting all-ceramic and metal-based reconstructions: Results at 18 months of loading. Int J Periodontics Restorative Dent. 2018; 38:173–179. PMID: 29447309.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Attitude and treatment options in implant-supported prosthetics: A survey among a cohort of German dentists
- Prospective clinical comparative evaluation of implant-supported zirconia-lithium disilicate bilayered ceramic and metalceramic posterior prostheses: a 3-year follow-up
- Full-mouth rehabilitation with pressed ceramic technique using provisional restorations
- The level of buccal gingival margin around single and two adjacent implant restorations: a preliminary result
- Mechanical Complications of Posterior Implant Restorations using CAD/CAM Titanium Customized Abutments: A Retrospective Study