Cancer Res Treat.
2019 Jan;51(1):24-33. 10.4143/crt.2017.404.
Incremental Role of Pancreatic Magnetic Resonance Imaging after Staging Computed Tomography to Evaluate Patients with Pancreatic Ductal Adenocarcinoma
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. RADPMS@yuhs.ac
- 2Department of Radiology, Yonsei Biomedical Research Institute, Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2437595
- DOI: http://doi.org/10.4143/crt.2017.404
Abstract
- PURPOSE
The purpose of this study was to investigate the impact of contrast enhanced pancreatic magnetic resonance imaging (MRI) in resectability and prognosis evaluation after staging computed tomography (CT) in patients with pancreatic ductal adenocarcinoma (PDA).
MATERIALS AND METHODS
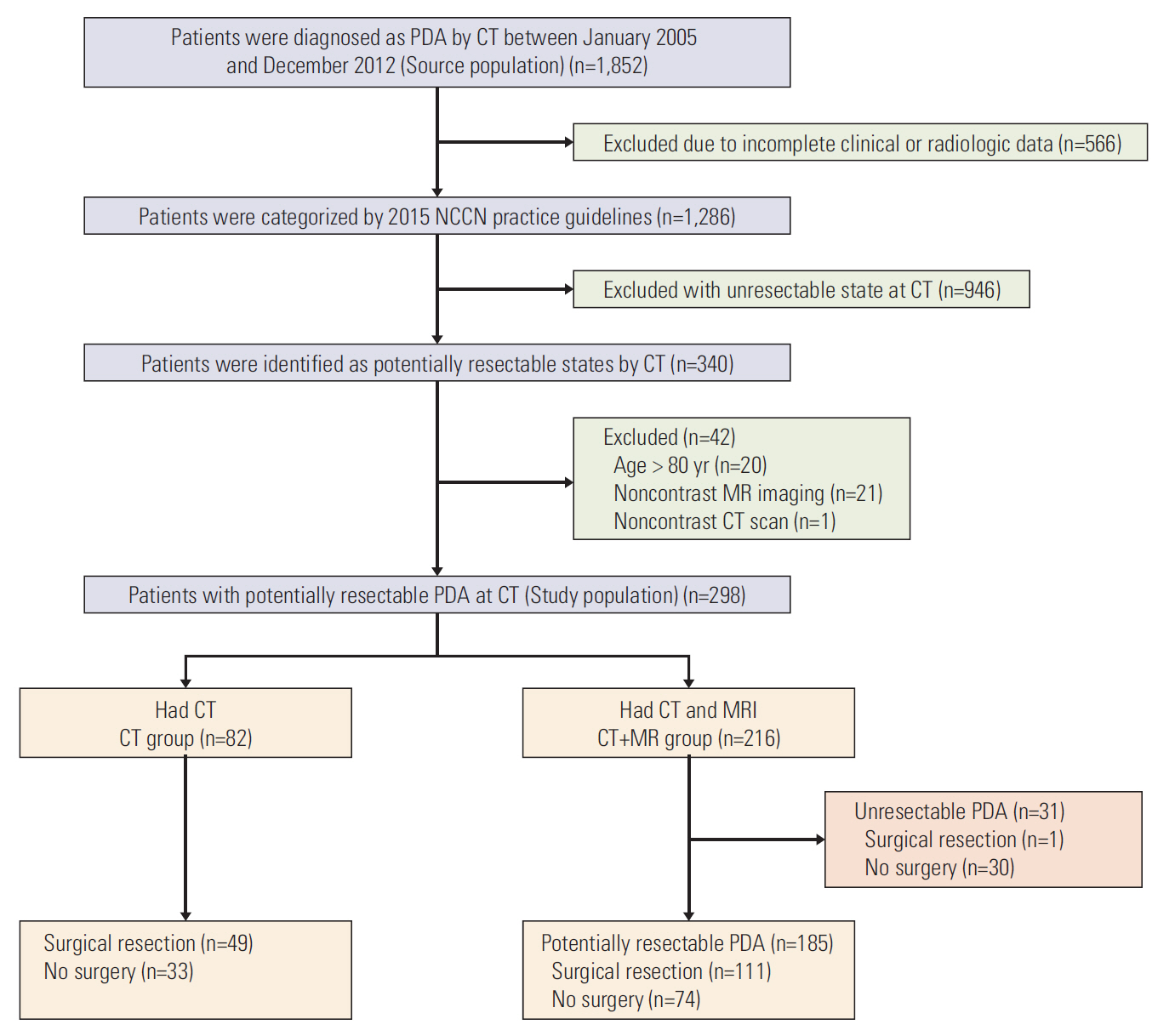
From January 2005 to December 2012, 298 patients were diagnosed to have potentially resectable stage PDA on CT. Patients were divided into CT+MR (patients underwent both CT and MRI; n=216) and CT only groups (n=82). Changes in resectability staging in the CT+MR group were evaluated. The overall survival was compared between the two groups. The recurrence-free survival and median time to liver metastasis after curative surgery were compared between the two groups.
RESULTS
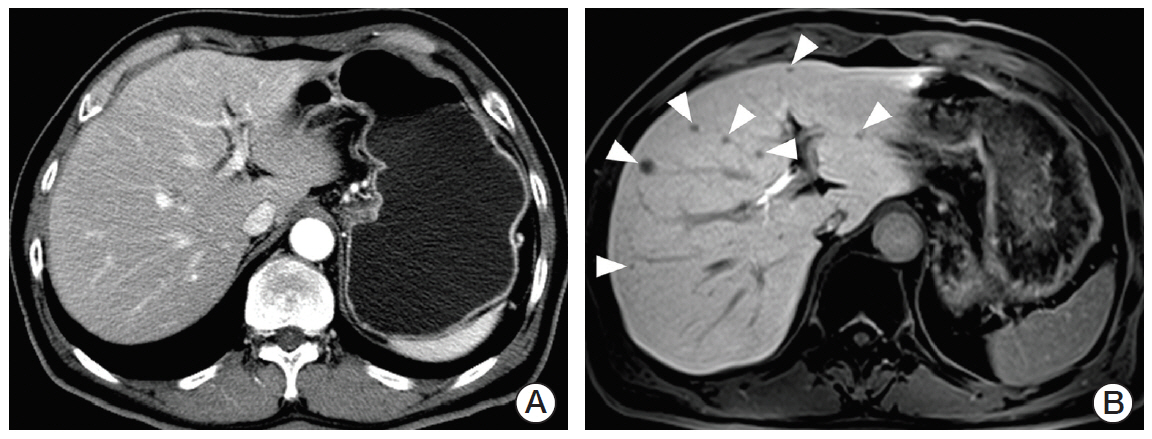
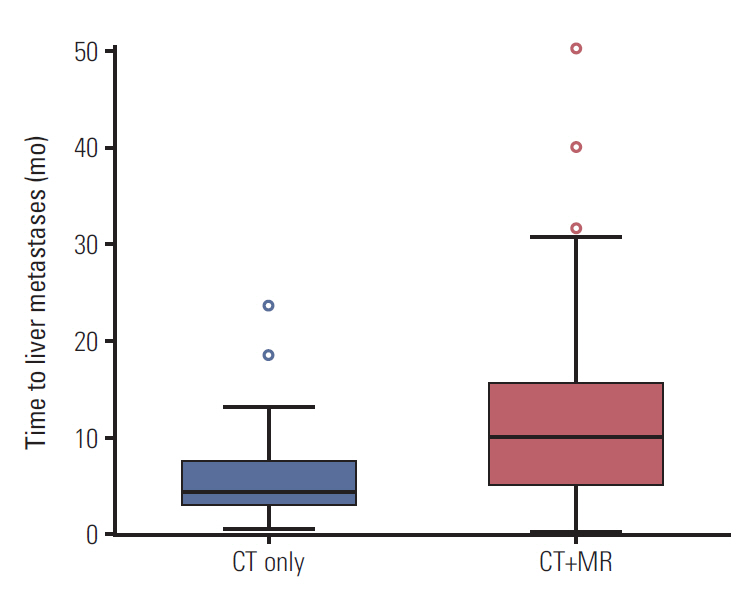
Staging was changed from resectable on CT to unresectable state on MRI in 14.4% of (31 of 216 patients) patients of the CT+MR group. The overall survival and recurrence-free survival rates were not significantly different between the two groups (p=0.162 and p=0.721, respectively). The median time to liver metastases after curative surgery in the CT+MR group (9.9 months) was significantly longer than that in the CT group (4.2 months) (p=0.011).
CONCLUSION
Additional MRI resulted in changes of resectability and treatment modifications in a significant proportion of patients who have potentially resectable state at CT and in prolonged time to liver metastases in patients after curative surgery. Additional MRI to standard staging CT can be recommended for surgical candidates of PDA.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29.
Article2. Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010; 7:e1000267.
Article3. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016; 388:73–85.
Article4. Orr RK. Outcomes in pancreatic cancer surgery. Surg Clin North Am. 2010; 90:219–34.
Article5. McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007; 246:246–53.6. Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009; 16:1727–33.
Article7. Raman SP, Horton KM, Fishman EK. Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J. 2012; 18:511–22.
Article8. Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol. 2014; 20:7864–77.
Article9. Tamburrino D, Riviere D, Yaghoobi M, Davidson BR, Gurusamy KS. Diagnostic accuracy of different imaging modalities following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev. 2016; 9:CD011515.
Article10. Egorov VI, Petrov RV, Solodinina EN, Karmazanovsky GG, Starostina NS, Kuruschkina NA. Computed tomography-based diagnostics might be insufficient in the determination of pancreatic cancer unresectability. World J Gastrointest Surg. 2013; 5:83–96.
Article11. Kim YE, Park MS, Hong HS, Kang CM, Choi JY, Lim JS, et al. Effects of neoadjuvant combined chemotherapy and radiation therapy on the CT evaluation of resectability and staging in patients with pancreatic head cancer. Radiology. 2009; 250:758–65.
Article12. National Comprehensive Cancer Network. Practice guidelines in oncology for pancreatic adenocarcinoma V2 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2015. [cited 2018 Feb 5]. Available from: http://www.nccn.org.professionals/physician_PDF/pancreatic.pdf.13. Kamisawa T, Isawa T, Koike M, Tsuruta K, Okamoto A. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas. 1995; 11:345–9.
Article14. Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008; 132:931–9.
Article15. Kim HJ, Lee SS, Byun JH, Kim JC, Yu CS, Park SH, et al. Incremental value of liver MR imaging in patients with potentially curable colorectal hepatic metastasis detected at CT: a prospective comparison of diffusion-weighted imaging, gadoxetic acid-enhanced MR imaging, and a combination of both MR techniques. Radiology. 2015; 274:712–22.
Article16. Scharitzer M, Ba-Ssalamah A, Ringl H, Kolblinger C, Grunberger T, Weber M, et al. Preoperative evaluation of colorectal liver metastases: comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur Radiol. 2013; 23:2187–96.
Article17. Muhi A, Ichikawa T, Motosugi U, Sou H, Nakajima H, Sano K, et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acidenhanced MRI. J Magn Reson Imaging. 2011; 34:326–35.
Article18. Motosugi U, Ichikawa T, Morisaka H, Sou H, Muhi A, Kimura K, et al. Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology. 2011; 260:446–53.
Article19. Han K, Park SH, Kim KW, Kim HJ, Lee SS, Kim JC, et al. Use of liver magnetic resonance imaging after standard staging abdominopelvic computed tomography to evaluate newly diagnosed colorectal cancer patients. Ann Surg. 2015; 261:480–6.
Article20. Chew C, O'Dwyer PJ. The value of liver magnetic resonance imaging in patients with findings of resectable pancreatic cancer on computed tomography. Singapore Med J. 2016; 57:334–8.
Article21. Holzapfel K, Reiser-Erkan C, Fingerle AA, Erkan M, Eiber MJ, Rummeny EJ, et al. Comparison of diffusion-weighted MR imaging and multidetector-row CT in the detection of liver metastases in patients operated for pancreatic cancer. Abdom Imaging. 2011; 36:179–84.
Article22. Koelblinger C, Ba-Ssalamah A, Goetzinger P, Puchner S, Weber M, Sahora K, et al. Gadobenate dimeglumine-enhanced 3.0-T MR imaging versus multiphasic 64-detector row CT: prospective evaluation in patients suspected of having pancreatic cancer. Radiology. 2011; 259:757–66.
Article23. Chen FM, Ni JM, Zhang ZY, Zhang L, Li B, Jiang CJ. Presurgical evaluation of pancreatic cancer: a comprehensive imagingcomparison of CT versus MRI. AJR Am J Roentgenol. 2016; 206:526–35.24. Bilici A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J Gastroenterol. 2014; 20:10802–12.
Article25. El-Rayes BF, Jasti P, Severson RK, Almhanna K, Philip PA, Shields A, et al. Impact of race, age, and socioeconomic status on participation in pancreatic cancer clinical trials. Pancreas. 2010; 39:967–71.
Article26. Kim SM, Eads JR. Adjuvant and neoadjuvant therapy for resectable pancreatic and periampullary cancer. Surg Clin North Am. 2016; 96:1287–300.
Article27. O'Reilly EM, Perelshteyn A, Jarnagin WR, Schattner M, Gerdes H, Capanu M, et al. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg. 2014; 260:142–8.28. Seo N, Park MS, Han K, Lee KH, Park SH, Choi GH, et al. Magnetic resonance imaging for colorectal cancer metastasis to the liver: comparative effectiveness research for the choice of contrast agents. Cancer Res Treat. 2018; 50:60–70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Diagnosis in Pancreatic Cancer
- The Role of Imaging in Current Treatment Strategies for Pancreatic Adenocarcinoma
- Survival rate according to stages of pancreatic cancer
- Congenital Variants and Anomalies of the Pancreas and Pancreatic Duct: Imaging by Magnetic Resonance Cholangiopancreaticography and Multidetector Computed Tomography
- Pancreas Neuroendocrine Tumor and Its Mimics: Review of Cross-Sectional Imaging Findings for Differential Diagnosis