Yeungnam Univ J Med.
2019 Jan;36(1):8-15. 10.12701/yujm.2019.00101.
Pathological interpretation of connective tissue disease-associated lung diseases
- Affiliations
-
- 1Department of Pathology, Dongkang Hospital, Ulsan, Korea. k19156ky@gmail.com
- KMID: 2434093
- DOI: http://doi.org/10.12701/yujm.2019.00101
Abstract
- Connective tissue diseases (CTDs) can affect all compartments of the lungs, including airways, alveoli, interstitium, vessels, and pleura. CTD-associated lung diseases (CTD-LDs) may present as diffuse lung disease or as focal lesions, and there is significant heterogeneity between the individual CTDs in their clinical and pathological manifestations. CTD-LDs may presage the clinical diagnosis a primary CTD, or it may develop in the context of an established CTD diagnosis. CTD-LDs reveal acute, chronic or mixed pattern of lung and pleural manifestations. Histopathological findings of diverse morphological changes can be present in CTD-LDs airway lesions (chronic bronchitis/bronchiolitis, follicular bronchiolitis, etc.), interstitial lung diseases (nonspecific interstitial pneumonia/fibrosis, usual interstitial pneumonia, lymphocytic interstitial pneumonia, diffuse alveolar damage, and organizing pneumonia), pleural changes (acute fibrinous or chronic fibrous pleuritis), and vascular changes (vasculitis, capillaritis, pulmonary hemorrhage, etc.). CTD patients can be exposed to various infectious diseases when taking immunosuppressive drugs. Histopathological patterns of CTD-LDs are generally nonspecific, and other diseases that can cause similar lesions in the lungs must be considered before the diagnosis of CTD-LDs. A multidisciplinary team involving pathologists, clinicians, and radiologists can adequately make a proper diagnosis of CTD-LDs.
Keyword
MeSH Terms
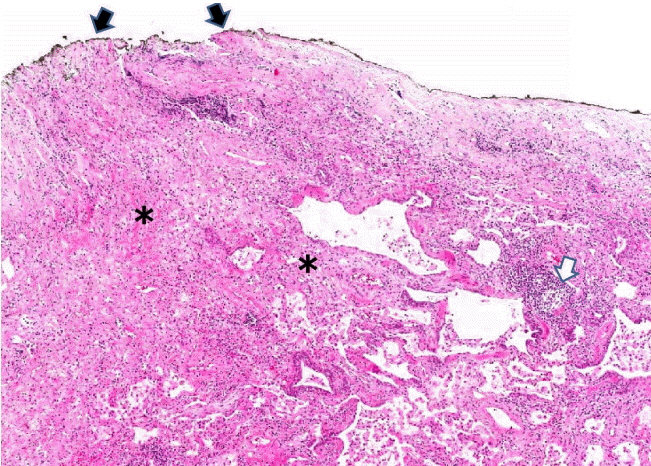
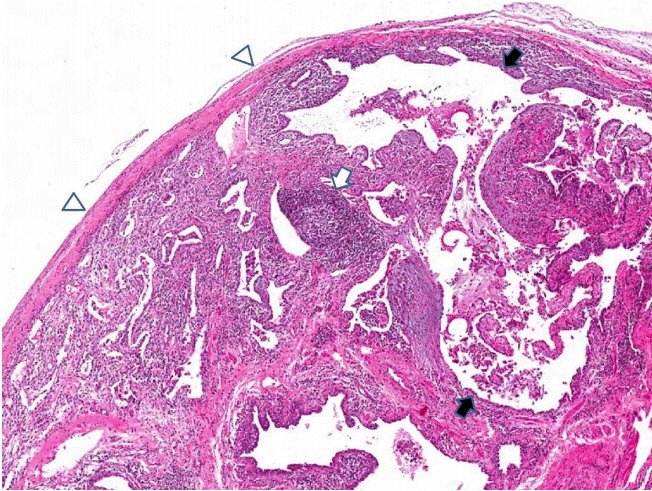
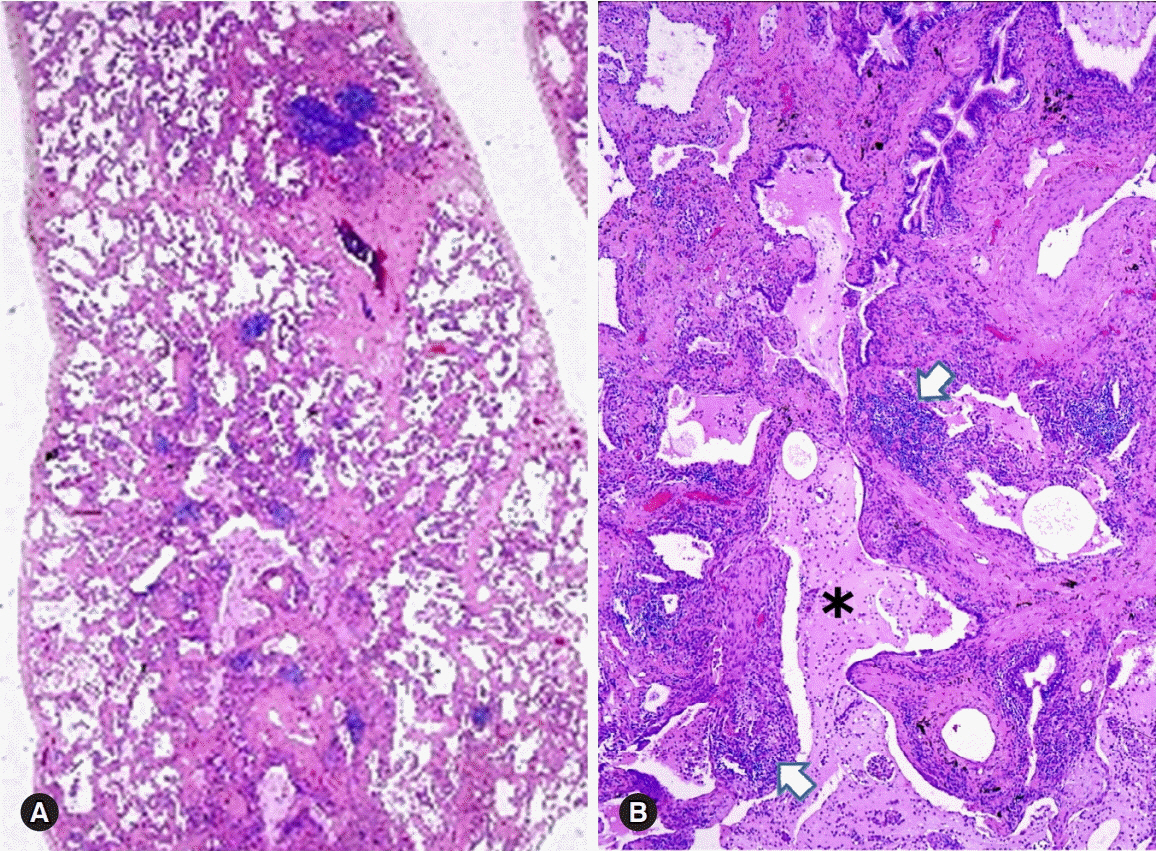
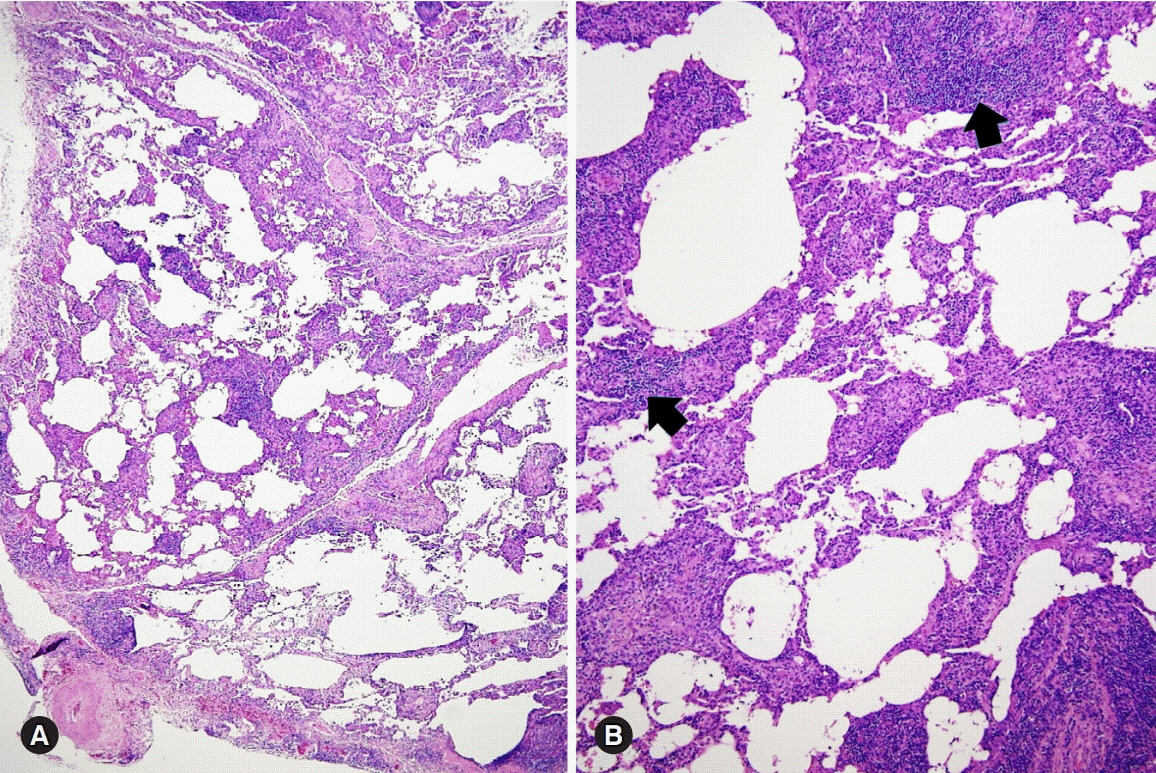
Figure
Reference
-
References
1. Vivero M, Padera RF. Histopathology of lung disease in the connective tissue diseases. Rheum Dis Clin North Am. 2015; 41:197–211.
Article2. Tansey D, Wells AU, Colby TV, Ip S, Nikolakoupolou A, du Bois RM, et al. Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosis. Histopathology. 2004; 44:585–96.
Article3. Cipriani NA, Strek M, Noth I, Gordon IO, Charbeneau J, Krishnan JA, et al. Pathologic quantification of connective tissue disease-associated versus idiopathic usual interstitial pneumonia. Arch Pathol Lab Med. 2012; 136:1253–8.
Article4. Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013; 188:733–48.
Article5. Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clin Chest Med. 2010; 31:501–12.
Article6. Hakala M, Pääkkö P, Huhti E, Tarkka M, Sutinen S. Open lung biopsy of patients with rheumatoid arthritis. Clin Rheumatol. 1990; 9:452–60.
Article7. Hashimoto K, Nakanishi H, Yamasaki A, Chikumi H, Hasegawa Y, Watanabe M, et al. Pulmonary findings without the influence of therapy in a patient with rheumatoid arthritis: an autopsy case. J Med Invest. 2007; 54:340–4.
Article8. Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985; 131:770–7.9. Yoshinouchi T, Ohtsuki Y, Fujita J, Yamadori I, Bandoh S, Ishida T, et al. Nonspecific interstitial pneumonia pattern as pulmonary involvement of rheumatoid arthritis. Rheumatol Int. 2005; 26:121–5.
Article10. Nakamura Y, Suda T, Kaida Y, Kono M, Hozumi H, Hashimoto D, et al. Rheumatoid lung disease: prognostic analysis of 54 biopsy-proven cases. Respir Med. 2012; 106:1164–9.
Article11. Song JW, Do KH, Kim MY, Jang SJ, Colby TV, Kim DS. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest. 2009; 136:23–30.
Article12. Yousem SA, Colby TV, Carrington CB. Follicular bronchitis/bronchiolitis. Hum Pathol. 1985; 16:700–6.
Article13. Hozumi H, Nakamura Y, Johkoh T, Sumikawa H, Colby TV, Kono M, et al. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open. 2013; 3:e003132.
Article14. Schneider F, Gruden J, Tazelaar HD, Leslie KO. Pleuropulmonary pathology in patients with rheumatic disease. Arch Pathol Lab Med. 2012; 136:1242–52.
Article15. Schwarz MI, Zamora MR, Hodges TN, Chan ED, Bowler RP, Tuder RM. Isolated pulmonary capillaritis and diffuse alveolar hemorrhage in rheumatoid arthritis and mixed connective tissue disease. Chest. 1998; 113:1609–15.
Article16. Fischer A, Swigris JJ, Groshong SD, Cool CD, Sahin H, Lynch DA, et al. Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest. 2008; 134:601–5.
Article17. Parra ER, Otani LH, de Carvalho EF, Ab'Saber A, Capelozzi VL. Systemic sclerosis and idiopathic interstitial pneumonia: histomorphometric differences in lung biopsies. J Bras Pneumol. 2009; 35:529–40.
Article18. Griffin MT, Robb JD, Martin JR. Diffuse alveolar haemorrhage associated with progressive systemic sclerosis. Thorax. 1990; 45:903–4.
Article19. Aggarwal N, Lopez R, Gabbard S, Wadhwa N, Devaki P, Thota PN. Spectrum of esophageal dysmotility in systemic sclerosis on high-resolution esophageal manometry as defined by Chicago classification. Dis Esophagus. 2017; 30:1–6.
Article20. Keane MP, Lynch JP 3rd. Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax. 2000; 55:159–66.21. Haupt HM, Moore GW, Hutchins GM. The lung in systemic lupus erythematosus. Analysis of the pathologic changes in 120 patients. Am J Med. 1981; 71:791–8.22. Matthay RA, Schwarz MI, Petty TL, Stanford RE, Gupta RC, Sahn SA, et al. Pulmonary manifestations of systemic lupus erythematosus: review of twelve cases of acute lupus pneumonitis. Medicine (Baltimore). 1975; 54:397–409.
Article23. Gammon RB, Bridges TA, al-Nezir H, Alexander CB, Kennedy JI Jr. Bronchiolitis obliterans organizing pneumonia associated with systemic lupus erythematosus. Chest. 1992; 102:1171–4.
Article24. Zamora MR, Warner ML, Tuder R, Schwarz MI. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore). 1997; 76:192–202.
Article25. Weinrib L, Sharma OP, Quismorio FP Jr. A long-term study of interstitial lung disease in systemic lupus erythematosus. Semin Arthritis Rheum. 1990; 20:48–56.
Article26. Fathi M, Lundberg IE. Interstitial lung disease in polymyositis and dermatomyositis. Curr Opin Rheumatol. 2005; 17:701–6.
Article27. Douglas WW, Tazelaar HD, Hartman TE, Hartman RP, Decker PA, Schroeder DR, et al. Polymyositis-dermatomyositis-associated interstitial lung disease. Am J Respir Crit Care Med. 2001; 164:1182–5.
Article28. Tazelaar HD, Viggiano RW, Pickersgill J, Colby TV. Interstitial lung disease in polymyositis and dermatomyositis. Clinical features and prognosis as correlated with histologic findings. Am Rev Respir Dis. 1990; 141:727–33.
Article29. Matsuki Y, Yamashita H, Takahashi Y, Kano T, Shimizu A, Itoh K, et al. Diffuse alveolar damage in patients with dermatomyositis: a six-case series. Mod Rheumatol. 2012; 22:243–8.
Article30. Schwarz MI, Sutarik JM, Nick JA, Leff JA, Emlen JW, Tuder RM. Pulmonary capillaritis and diffuse alveolar hemorrhage. A primary manifestation of polymyositis. Am J Respir Crit Care Med. 1995; 151:2037–40.
Article31. Shi JH, Liu HR, Xu WB, Feng RE, Zhang ZH, Tian XL, et al. Pulmonary manifestations of Sjögren's syndrome. Respiration. 2009; 78:377–86.32. Ito I, Nagai S, Kitaichi M, Nicholson AG, Johkoh T, Noma S, et al. Pulmonary manifestations of primary Sjogren's syndrome: a clinical, radiologic, and pathologic study. Am J Respir Crit Care Med. 2005; 171:632–8.
Article33. Kokosi M, Riemer EC, Highland KB. Pulmonary involvement in Sjögren syndrome. Clin Chest Med. 2010; 31:489–500.
Article34. Hant FN, Herpel LB, Silver RM. Pulmonary manifestations of scleroderma and mixed connective tissue disease. Clin Chest Med. 2010; 31:433–49.
Article35. Bryson T, Sundaram B, Khanna D, Kazerooni EA. Connective tissue disease-associated interstitial pneumonia and idiopathic interstitial pneumonia: similarity and difference. Semin Ultrasound CT MR. 2014; 35:29–38.
Article36. Bull TM, Fagan KA, Badesch DB. Pulmonary vascular manifestations of mixed connective tissue disease. Rheum Dis Clin North Am. 2005; 31:451–64.
Article37. Aubry MC. Necrotizing granulomatous inflammation: what does it mean if your special stains are negative? Mod Pathol. 2012; 25(Suppl 1):S31–8.
Article38. Myers JL, Limper AH, Swensen SJ. Drug-induced lung disease: a pragmatic classification incorporating HRCT appearances. Semin Respir Crit Care Med. 2003; 24:445–54.
Article39. Carrillo J, Restrepo CS, Rosado de Christenson M, Ojeda Leon P, Lucia Rivera A, Koss MN. Lymphoproliferative lung disorders: a radiologic-pathologic overview. Part I: reactive disorders. Semin Ultrasound CT MR. 2013; 34:525–34.
Article40. Kono M, Nakamura Y, Enomoto N, Hashimoto D, Fujisawa T, Inui N, et al. Usual interstitial pneumonia preceding collagen vascular disease: a retrospective case control study of patients initially diagnosed with idiopathic pulmonary fibrosis. PLoS One. 2014; 9:e94775.
Article41. de Lauretis A, Veeraraghavan S, Renzoni E. Connective tissue disease-associated interstitial lung disease: how does it differ from IPF? How should the clinical approach differ? Chron Respir Dis. 2011; 8:53–82.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interstitial Lung Diseases: Respiratory Review of 2013
- Connective Tissue Disease-Associated Interstitial Lung Disease
- A Case of Trigeminal Neuropathy Associated with Mixed Connective Tissue Disease
- Early Detection of Pulmonary Hypertension in Connective Tissue Disease
- Pleural Effusion in Connective Tissue Diseases