Chonnam Med J.
2019 Jan;55(1):47-53. 10.4068/cmj.2019.55.1.47.
Adherence to the GOLD Guideline in COPD Management of South Korea: Findings from KOCOSS Study 2011–2018
- Affiliations
-
- 1Division of Pulmonary Medicine, Department of Internal Medicine, Chonnam National University Hospital, Gwangju, Korea. lscmd@jnu.ac.kr
- 2Department of Internal Medicine, The Catholic University of Korea, Seoul St. Mary's Hospital, Seoul, Korea.
- 3Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Division of Pulmonary and Critical Care Medicine, Department of Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine and the Clinical Research Center for Chronic Obstructive Airway Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 6Division of Pulmonary Medicine, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University Medical School, Anyang, Korea.
- 7Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 8Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 2432255
- DOI: http://doi.org/10.4068/cmj.2019.55.1.47
Abstract
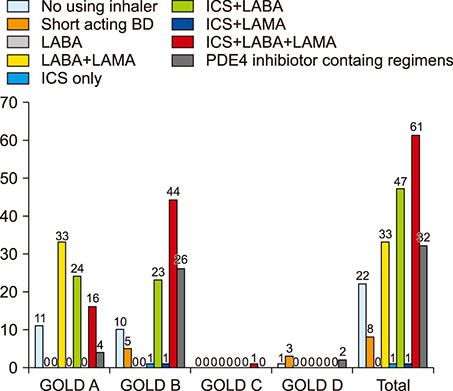
- The guidelines for chronic obstructive pulmonary disease (COPD) treatment are important for the management of the disease. However, studies regarding the treatment adherence to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have been scarce in Korea. Therefore, to examine the adherence to the GOLD guidelines, we examined the patterns of prescribed medication in COPD patients from 2011 to 2018. Patients were classified as having been appropriately and inappropriately treated (overtreatment or undertreatment) for the GOLD group. Appropriate medical therapy was defined as using the first choice or alternative choice drug recommended in the GOLD guidelines. Inappropriate therapy was classified as overtreatment or undertreatment in accordance with the categorization in the GOLD guidelines. According to treatment of 2011 GOLD guidelines, there was inappropriate treatment in 52.3% in group A, 47.3% in group B, 56.3% in group C, and 17.8% in group D. According to treatment of 2017 GOLD guidelines, there was inappropriate treatment in 66.7% in group A, 45.3% in group B, 14.3% in group C, and 24.0% in group D. The common type of inappropriate COPD treatment is overtreatment, with inhaled corticosteroid (ICS) containing regimens. In conclusions, adherence to the GOLD guideline by the pulmonologist in clinical practice is still low in Korea. Therefore, we need better strategies to both optimize the use of the guidelines and adhere to the guidelines as well.
Figure
Reference
-
1. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017; 195:557–582.
Article2. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187:347–365.
Article3. Turan O, Emre JC, Deniz S, Baysak A, Turan PA, Mirici A. Adherence to current COPD guidelines in Turkey. Expert Opin Pharmacother. 2016; 17:153–158.
Article4. Palmiotti GA, Lacedonia D, Liotino V, Schino P, Satriano F, Di Napoli PL, et al. Adherence to GOLD guidelines in real-life COPD management in the Puglia region of Italy. Int J Chron Obstruct Pulmon Dis. 2018; 13:2455–2462.
Article5. Jochmann A, Scherr A, Jochmann DC, Miedinger D, Török SS, Chhajed PN, et al. Impact of adherence to the GOLD guidelines on symptom prevalence, lung function decline and exacerbation rate in the Swiss COPD cohort. Swiss Med Wkly. 2012; 142:w13567.
Article6. Chan KP, Ko FW, Chan HS, Wong ML, Mok TY, Choo KL, et al. Adherence to a COPD treatment guideline among patients in Hong Kong. Int J Chron Obstruct Pulmon Dis. 2017; 12:3371–3379.
Article7. Lee JY, Chon GR, Rhee CK, Kim DK, Yoon HK, Lee JH, et al. Characteristics of patients with chronic obstructive pulmonary disease at the first visit to a pulmonary medical center in Korea: the Korea COPD Subgroup Study Team Cohort. J Korean Med Sci. 2016; 31:553–560.
Article8. Ghosh S, Anderson WH, Putcha N, Han MK, Curtis JL, Criner GJ, et al. Alignment of inhaled COPD therapies with published strategies: analysis of the GOLD recommendations in SPIROMICS. Ann Am Thorac Soc. 2018; DOI: 10.1513/AnnalsATS.201804-283OC. [Epub ahead of print].9. White P, Thornton H, Pinnock H, Georgopoulou S, Booth HP. Overtreatment of COPD with inhaled corticosteroids--implications for safety and costs: cross-sectional observational study. PLoS One. 2013; 8:e75221.10. Park YB, Rhee CK, Yoon HK, Oh YM, Lim SY, Lee JH, et al. Revised (2018) COPD clinical practice guideline of the Korean Academy of Tuberculosis and Respiratory Disease: a summary. Tuberc Respir Dis (Seoul). 2018; 81:261–273.
Article11. Calzetta L, Rogliani P, Matera MG, Cazzola M. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest. 2016; 149:1181–1196.
Article12. Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016; 374:2222–2234.
Article13. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:775–789.
Article14. Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013; 1:524–533.
Article15. Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008; 177:19–26.
Article16. Corrado A, Rossi A. How far is real life from COPD therapy guidelines? An Italian observational study. Respir Med. 2012; 106:989–997.
Article17. Jebrak G. [COPD routine management in France: are guidelines used in clinical practice?]. Rev Mal Respir. 2010; 27:11–18. French.18. Watz H, Tetzlaff K, Wouters EF, Kirsten A, Magnussen H, Rodriguez-Roisin R, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016; 4:390–398.
Article19. Barnes NC, Sharma R, Lettis S, Calverley PM. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J. 2016; 47:1374–1382.
Article20. Plaza V, Álvarez F, Calle M, Casanova C, Cosío BG, López-Viña A, et al. Consensus on the Asthma-COPD Overlap (ACO) between the Spanish COPD Guidelines (GesEPOC) and the Spanish Guidelines on the Management of Asthma (GEMA). Arch Bronconeumol. 2017; 53:443–449.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adherence to Pharmacological Management Guidelines for Stable Chronic Obstructive Lung Disease
- Blood eosinophil count and treatment patterns of chronic obstructive pulmonary disease patients in South Korea using real-world data
- Comparison of Korean COPD Guideline and GOLD Initiative Report in Term of Acute Exacerbation: A Validation Study for Korean COPD Guideline
- Guideline adherence to chemotherapy administration safety standards: a survey on nurses in a single institute
- Prescription Patterns and Factors Related to the Number of Medications in Chronic Obstructive Pulmonary Disease in Non-elderly Adults