J Korean Ophthalmol Soc.
2019 Jan;60(1):1-8. 10.3341/jkos.2019.60.1.1.
Effect of Ultrasound-guided Hyaluronic Acid Filler Injection in Anophthalmic Enophthalmos Syndrome
- Affiliations
-
- 1Department of Ophthalmology, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea. oculoplasty@gmail.com
- 2T2B Infrastructure Center for Ocular Disease, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- KMID: 2431833
- DOI: http://doi.org/10.3341/jkos.2019.60.1.1
Abstract
- PURPOSE
To evaluate the outcomes of ultrasound-guided hyaluronic acid filler injection in anophthalmic enophthalmos syndrome patients.
METHODS
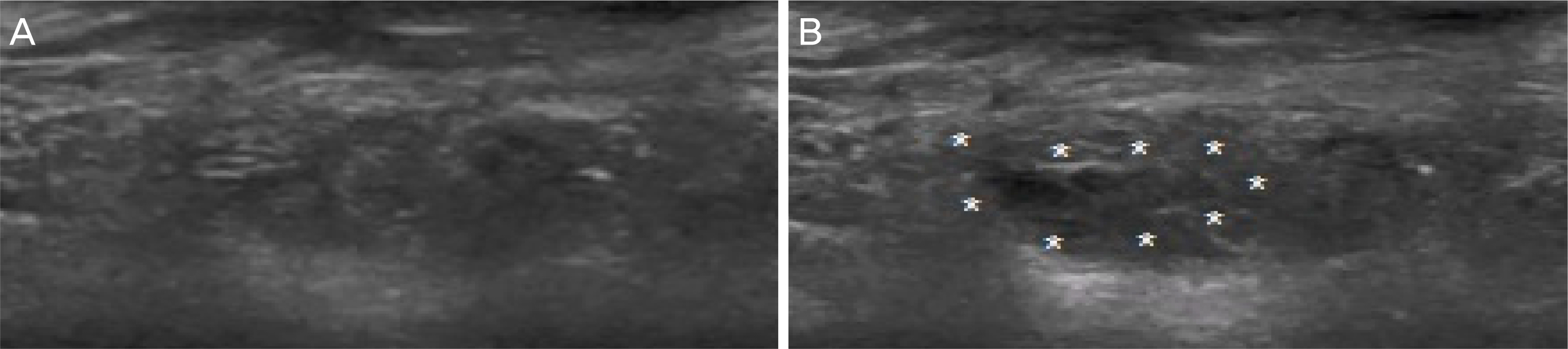
We retrospectively reviewed the clinical records of 14 patients who were diagnosed with anophthalmic enophthalmos syndrome and treated with ultrasound-guided hyaluronic acid filler injection from October 2011 to September 2017. Filler was injected transcutaneous in the retrobulbar area or eyelid under ultrasound guidance and improvement of superior deep sulcus and patient satisfaction after three months of injection were evaluated.
RESULTS
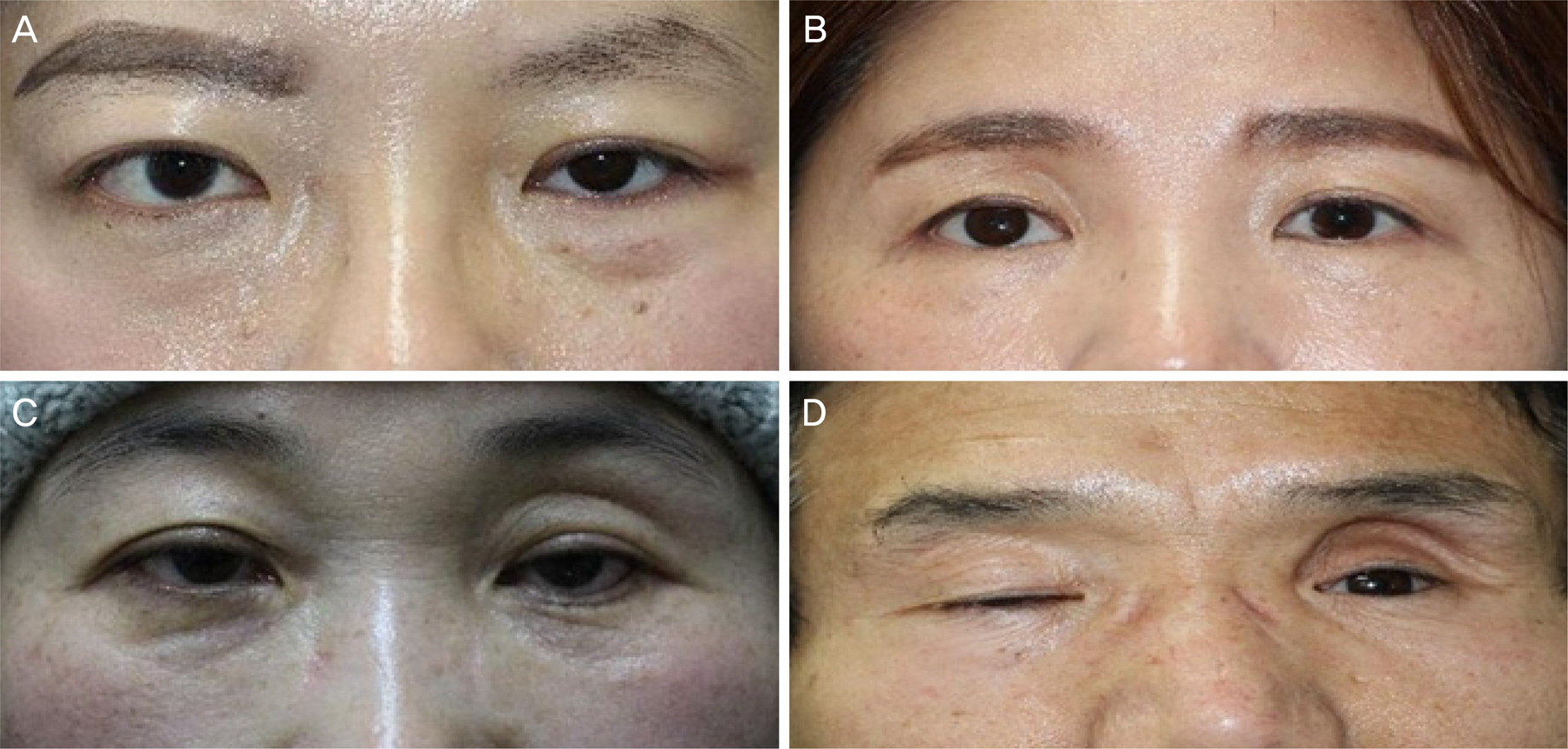
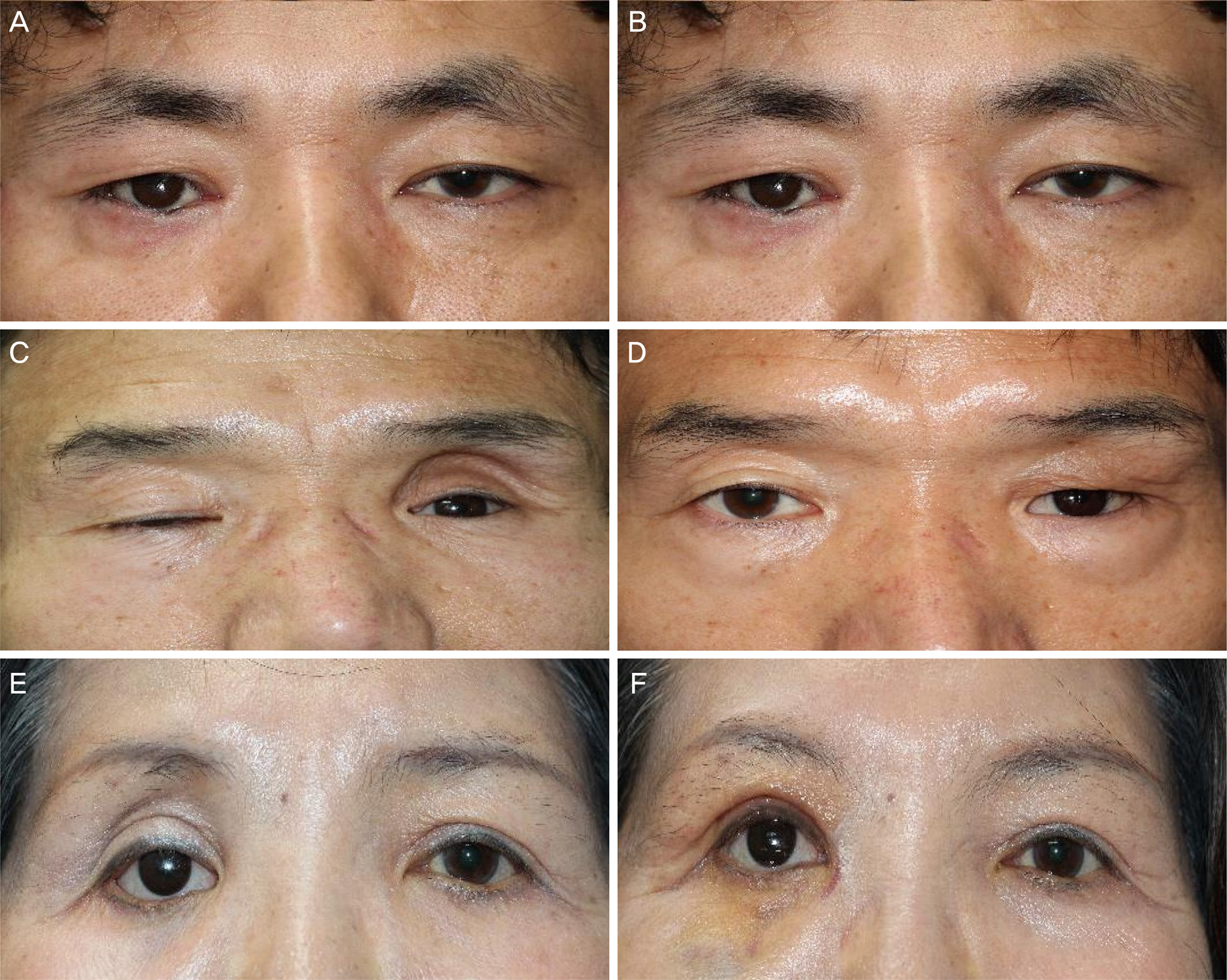
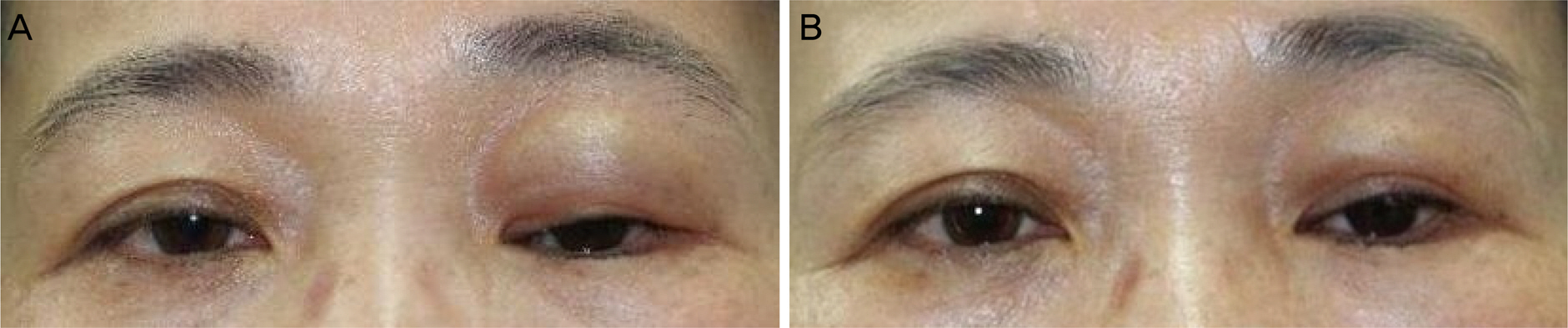
The mean number of injections was 1.2 ± 0.4, and 1.14 ± 0.30 mL of hyaluronic acid filler was used for each injection. There was a significant decrease in mean superior deep sulcus grade from 3.06 ± 0.90 before injection to 0.20 ± 0.56 after injection. Patient satisfaction was higher in the severe superior deep sulcus before injection group who showed greater superior deep sulcus improvement.
CONCLUSIONS
Hyaluronic acid filler injections for patients with anophthalmic enophthalmos syndrome were easy to perform in the outpatient department and showed excellent outcomes. The more severe the enophthalmos, the higher the satisfaction after injection, which showed that hyaluronic acid filler injection was an effective treatment for patients with severe enophthalmos syndrome.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Kaltreider SA, Shields MD, Hippeard SC, Patrie J. Anophthalmic ptosis: investigation of the mechanisms and statistical analysis. Ophthalmic Plast Reconstr Surg. 2003; 19:421–8.
Article2. Kaltreider SA, Lucarelli MJ. A simple algorithm for selection of implant size for enucleation and evisceration: a prospective study. Ophthalmic Plast Reconstr Surg. 2002; 18:336–41.3. Iverson RE, Vistnes LM, Siegel RJ. Correction of enophthalmos in the anophthalmic orbit. Plast Reconstr Surg. 1973; 51:545–54.
Article4. Meleca RJ, Mathog RH. Bone graft implantation for correction of the anophthalmic orbit. Arch Otolaryngol Head Neck Surg. 1994; 120:49–55.
Article5. Nasr AM, Jabak MH, Batainah Y. Orbital volume augmentation with subperiosteal room-temperature-vulcanized silicone implants: a clinical and histopathologic study. Ophthalmic Plast Reconstr Surg. 1994; 10:11–21. discussion 22–3.6. Paris GL, Spohn WG. Correction of enophthalmos in the abdominal orbit. Ophthalmology. 1980; 87:1301–8.7. Tyers AG, Collin JR. Orbital implants and post enucleation socket syndrome. Trans Ophthalmol Soc U K. 1982; 102(Pt 1):90–2.8. Vagefi MR, McMullan TF, Burroughs JR, et al. Orbital augmentation with injectable calcium hydroxylapatite for correction of post-enucleation/evisceration socket syndrome. Ophthalmic Plast Reconstr Surg. 2011; 27:90–4.
Article9. Cahill KV, Burns JA. Volume augmentation of the anophthalmic orbit with cross-linked collagen (Zyplast). Arch Ophthalmol. 1989; 107:1684–6.
Article10. Sergott TJ, Vistnes LM. Correction of enophthalmos and superior sulcus depression in the anophthalmic orbit: a long-term follow-up. Plast Reconstr Surg. 1987; 79:331–8.11. Hornblass A, Biesman BS, Eviatar JA. Current techniques of abdominal: a survey of 5,439 intraorbital implants and a review of the literature. Ophthalmic Plast Reconstr Surg. 1995; 11:77–86. discussion 87–8.12. Hunter PD, Baker SS. The treatment of enophthalmos by orbital abdominal of fat autograft. Arch Otolaryngol Head Neck Surg. 1994; 120:835–9.13. Hill JC, Savar D. Silicone augmentation of the enophthalmic socket. A 14 year review. Can J Ophthalmol. 1978; 13:294–8.14. da Silva AL, Bredemeier M, Gebrim ES, Moura Eda M. Intraorbital polyacrylamide gel injection for the treatment of anophthalmic enophthalmos. Ophthalmic Plast Reconstr Surg. 2008; 24:367–71.
Article15. Baumann L, Kaufman J, Saghari S. Collagen fillers. Dermatol Ther. 2006; 19:134–40.
Article16. Matarasso SL. The use of injectable collagens for aesthetic rejuvenation. Semin Cutan Med Surg. 2006; 25:151–7.
Article17. Duffy DM. Complications of fillers: overview. Dermatol Surg. 2005; 31(11 Pt 2):1626–33.
Article18. Prather CL, Jones DH. Liquid injectable silicone for soft tissue augmentation. Dermatol Ther. 2006; 19:159–68.
Article19. Danesh-Meyer HV, Savino PJ, Sergott RC. Case reports and small case series: ocular and cerebral ischemia following facial injection of autologous fat. Arch Ophthalmol. 2001; 119:777–8.20. Kotlus BS, Dryden RM. Correction of anophthalmic enophthalmos with injectable calcium hydroxylapatite (Radiesse). Ophthalmic Plast Reconstr Surg. 2007; 23:313–4.
Article21. Malhotra R. Deep orbital Sub-Q restylane (nonanimal stabilized hyaluronic acid) for orbital volume enhancement in sighted and anophthalmic orbits. Arch Ophthalmol. 2007; 125:1623–9.
Article22. Vagefi MR, McMullan TF, Burroughs JR, et al. Injectable calcium hydroxylapatite for orbital volume augmentation. Arch Facial Plast Surg. 2007; 9:439–42.
Article23. Jung SI, Kwon JW, Jung JH. Treatment of lower eyelid swelling abdominal retrobulbar hyaluronic acid filler injection in phthisis bulbi. J Korean Ophthalmol Soc. 2015; 56:1961–4.24. Quezada-Gaón N, Wortsman X. Ultrasound-guided hyaluronidase injection in cosmetic complications. J Eur Acad Dermatol Venereol. 2016; 30:e39–40.
Article25. Buchanan AG, Holds JB, Vagefi MR, et al. Anterior filler abdominal following injection of calcium hydroxylapatite gel (Radiesse) for anophthalmic orbital volume augmentation. Ophthalmic Plast Reconstr Surg. 2012; 28:335–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hyaluronidase: An overview of its properties, applications, and side effects
- Efficacy of Retrobulbar Filler Injections for Correcting Enophthalmos Associated with Phthisis Bulbi or Anophthalmos
- Facial Pseudocyst Caused by Hyaluronic Acid Filler Injection: A Case Report
- Treatment of Lower Eyelid Swelling after Retrobulbar Hyaluronic Acid Filler Injection in Phthisis Bulbi
- The Stability of the ALSA Plasma Gel Filler and the Hyaluronic Acid Filler in Rabbits