Urogenit Tract Infect.
2018 Dec;13(3):45-50. 10.14777/uti.2018.13.3.45.
Moving towards Evidence-Based Clinical Practice Guidelines
- Affiliations
-
- 1Department of Urology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 2Institute of Evidence-Based Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Argentine Cochrane Centre, Instituto Universitario Hospital Italiano, Buenos Aires, Argentina.
- 4Family and Community Medicine Service, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina.
- 5Urology Section, Minneapolis Veterans Healthcare System, Minneapolis, MN, USA. pdahm@umn.edu
- 6Department of Urology, University of Minnesota, Minneapolis, MN, USA.
- KMID: 2431247
- DOI: http://doi.org/10.14777/uti.2018.13.3.45
Abstract
- The Institute of Medicine in its report "Clinical Practice Guidelines we can trust" defined standards for clinical practice guidelines. However, many guidelines continue to rely on expert opinion and lack a formal framework for moving from evidence to recommendations. These guidelines may or may not be labeled as "consensus statements" and do not meet contemporary standards for guideline documents we would refer to as "evidence-based". Therefore, the Grading of Recommendations Assessment, Development and Evaluation working group developed a novel, rigorous and transparent approach to grading certainty (quality) of evidence. In addition, it created a system for "moving from evidence to decisions", for example for the development of evidence-based guidelines. In this article, we aim to introduce this approach to appraising the certainty of relevant evidence and estimate the benefits and detriments of health care interventions within the larger context of evidence-based medicine.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Introduction to the GRADE Approach for Evidence-Based Clinical Practice Guideline Development
Eu Chang Hwang, Jae Hung Jung
Urogenit Tract Infect. 2019;14(1):26-27. doi: 10.14777/uti.2019.14.1.26.
Reference
-
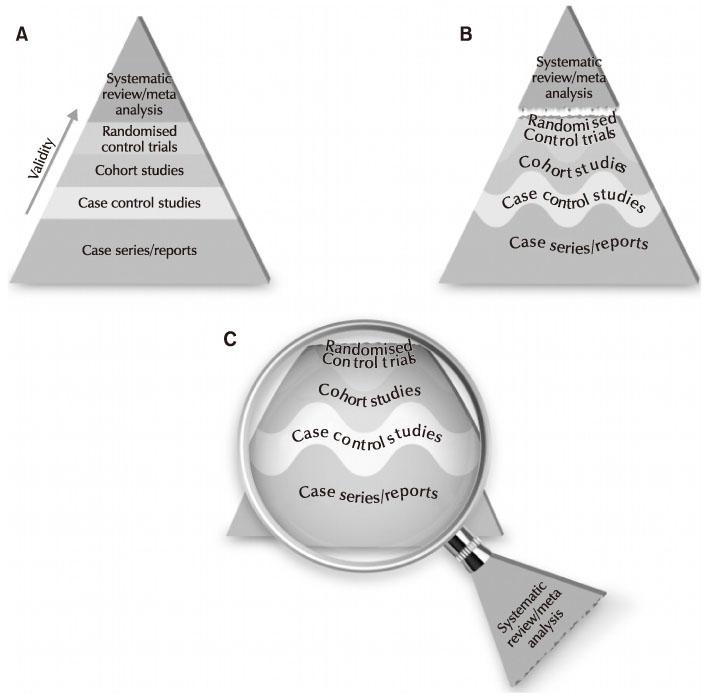
1. Korea Centers for Disease Control. Guidelines for the antibiotic use in urinary tract infections [Internet]. Cheongju: Korea Centers for Disease Control;2018. cited 2018 Sep 24. Available from: http://www.cdc.go.kr/CDC/together/CdcKrTogether0302.jsp?menuIds=HOME006-MNU2804-MNU3027-MNU2979&cid=138017.2. Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med. 2016; 21:125–127.
Article3. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ;. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008; 336:995–998.
Article4. Zimerman AL. Evidence-based medicine: a short history of a modern medical movement. Virtual Mentor. 2013; 15:71–76.
Article5. Guyatt GH. Evidence-based medicine. ACP J Club. 1991; 114:A16.6. Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. 1996. Clin Orthop Relat Res. 2007; 455:3–5.7. Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992; 268:2420–2425.8. Sackett DL, Strauss SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-based medicine: how to practice and teach EBM. 2nd ed. Edinburgh: Churchill Livingstone;2000.9. Graham R. Institute of Medicine. Clinical practice guidelines we can trust. Washington, DC: National Academies Press;2011.10. Siering U, Eikermann M, Hausner E, Hoffmann-Eßer W, Neugebauer EA. Appraisal tools for clinical practice guidelines: a systematic review. PLoS One. 2013; 8:e82915.
Article11. Ioannidis JP. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. 2016; 94:485–514.
Article12. Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews [Internet]. London: Cochrane;2018. cited 2018 Sep 24. Available from: https://community.cochrane.org/mecirmanual/introduction-key-points.13. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017; 358:j4008.
Article14. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339:b2535.
Article15. Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016; 69:225–234.
Article16. Han JL, Gandhi S, Bockoven CG, Narayan VM, Dahm P. The landscape of systematic reviews in urology (1998 to 2015): an assessment of methodological quality. BJU Int. 2017; 119:638–649.
Article17. Guyatt G, Meade M. What is evidence-based medicine?. In : Guyatt G, Rennie D, Meade M, Cook D, editors. Users' guides to the medical literature. Essentials of evidence-based clinical practice. 3rd ed. New York: McGraw-Hill education;2015. p. 16–18.18. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336:924–926.
Article19. Alonso-Coello P, Schunemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: introduction. BMJ. 2016; 353:i2016.
Article20. Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011; 64:380–382.
Article21. Guyatt G, Eikelboom JW, Akl EA, Crowther M, Gutterman D, Kahn SR, et al. A guide to GRADE guidelines for the readers of JTH. J Thromb Haemost. 2013; 11:1603–1608.
Article22. Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. GRADE Working Group. Going from evidence to recommendations. BMJ. 2008; 336:1049–1051.
Article23. Schunemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al. GRADE Working Group. GRADE guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol. 2016; 76:89–98.
Article24. McCormack J, Elwyn G. Shared decision is the only outcome that matters when it comes to evaluating evidence-based practice. BMJ Evid Based Med. 2018; 23:137–139.
Article25. Tikkinen KAO, Dahm P, Lytvyn L, Heen AF, Vernooij RWM, Siemieniuk RAC, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a clinical practice guideline. BMJ. 2018; 362:k3581.
Article26. Vernooij RWM, Lytvyn L, Pardo-Hernandez H, Albarqouni L, Canelo-Aybar C, Campbell K, et al. Values and preferences of men for undergoing prostate-specific antigen screening for prostate cancer: a systematic review. BMJ Open. 2018; 8:e025470.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nurses' Usage of Clinical Practice Guideline and Demand of Evidence Based Clinical Practice Guideline
- Development and Implementation of Clinical Practice Guidelines: Current Status in Korea
- Belief in Evidence-Based Practice, Awareness of Importance and Performance of Nursing Practice Guidelines among Novice Nurses and Preceptors in a Tertiary General Hospital
- Academic Strategies based on Evidence-Practice Gaps
- Changes in 2015 Canadian Clinical Practice Guidelines