Korean Circ J.
2019 Jan;49(1):69-80. 10.4070/kcj.2018.0097.
Safety and Efficacy of Biodegradable Polymer-biolimus-eluting Stents (BP-BES) Compared with Durable Polymer-everolimus-eluting Stents (DP-EES) in Patients Undergoing Complex Percutaneous Coronary Intervention
- Affiliations
-
- 1Division of Cardiology, Cardiovascular Center, Mediplex Sejong General Hospital, Incheon, Korea.
- 2Division of Cardiology, Department of Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jyhahn@skku.edu
- 3Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea.
- 4Division of Cardiology, Dongsuwon General Hospital, Suwon, Korea.
- 5Division of Cardiology, Department of Internal Medicine, Chung-Ang University Hospital, Seoul, Korea.
- 6Division of Cardiology, Department of Internal Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea. mccoyojh@gmail.com
- KMID: 2430749
- DOI: http://doi.org/10.4070/kcj.2018.0097
Abstract
- BACKGROUND AND OBJECTIVES
There are no data comparing clinical outcomes of complex percutaneous coronary intervention (PCI) between biodegradable polymer-biolimus-eluting stents (BP-BES) and durable polymer-everolimus-eluting stents (DP-EES). We sought to evaluate the safety and efficacy of BP-BES compared with DP-EES in patients undergoing complex PCI.
METHODS
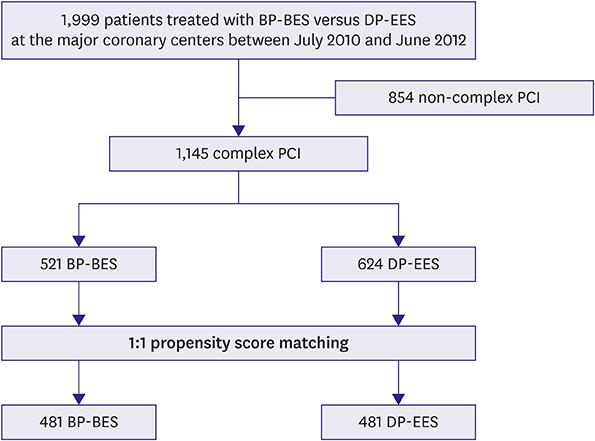
Patients enrolled in the SMART-DESK registry were stratified into 2 categories based on the complexity of PCI. Complex PCI was defined as having at least one of the following features: unprotected left main lesion, ≥2 lesions treated, total stent length >40 mm, minimal stent diameter ≤2.5 mm, or bifurcation as target lesion. The primary outcome was target lesion failure (TLF), defined as a composite of cardiac death, target vessel-related myocardial infarction (TV-MI), or target lesion revascularization (TLR) at 2 years of follow-up.
RESULTS
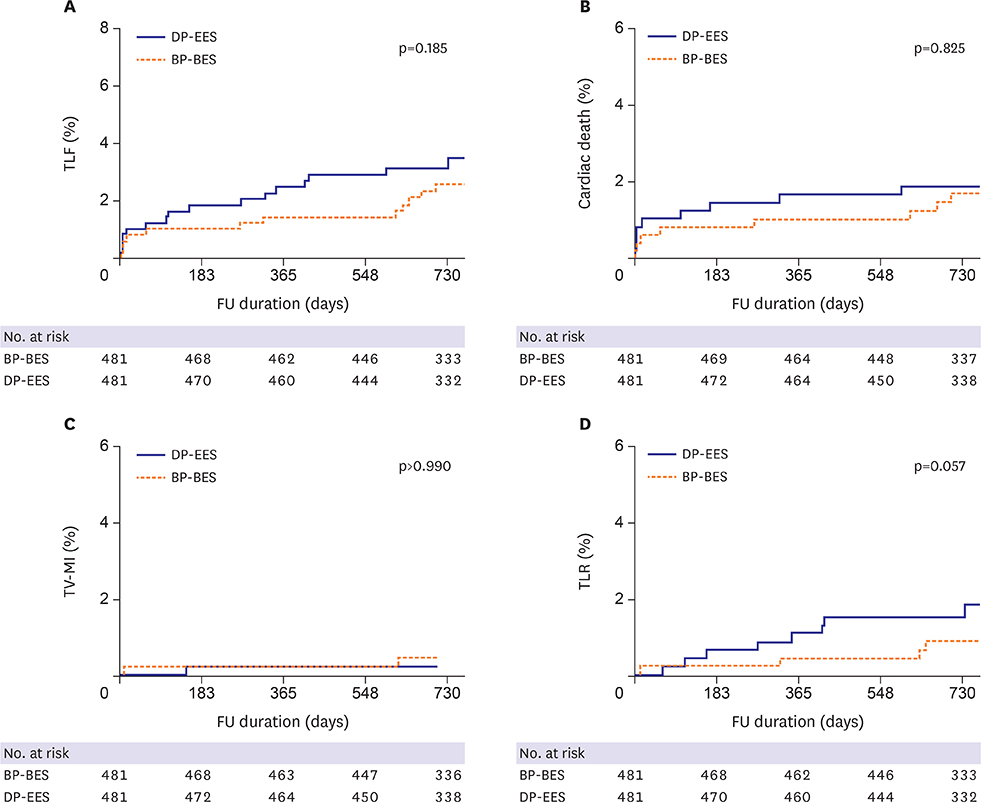
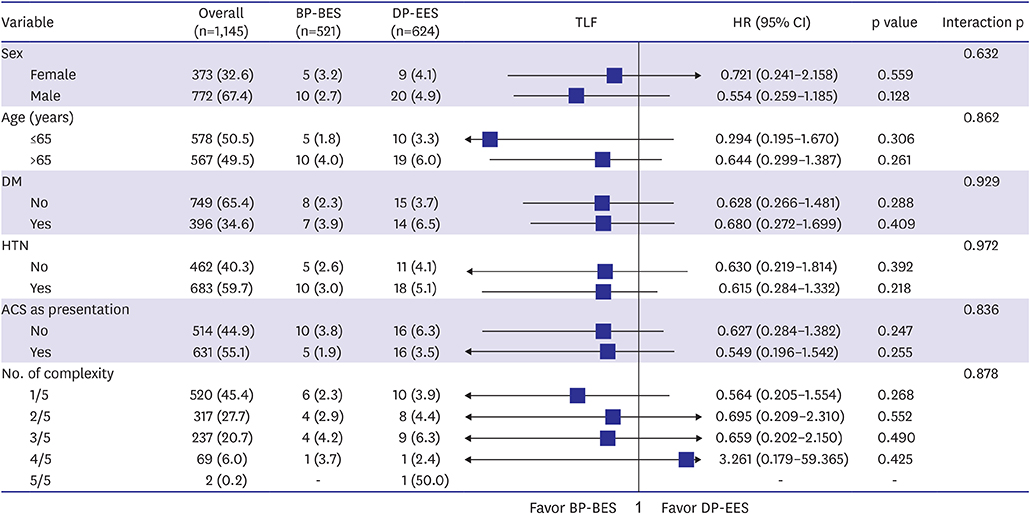
Of 1,999 patients, 1,145 (57.3%) underwent complex PCI: 521 patients were treated with BP-BES and 624 with DP-EES. In propensity-score matching analysis (481 pairs), the risks of TLF (3.8% vs. 5.2%, adjusted hazard ratio [HR], 0.578; 95% confidence interval [CI], 0.246-1.359; p=0.209), cardiac death (2.5% vs. 2.5%, adjusted HR, 0.787; 95% CI, 0.244-2.539; p=0.689), TV-MI (0.5% vs. 0.4%, adjusted HR, 1.128; 95% CI, 0.157-8.093; p=0.905), and TLR (1.1% vs. 2.9%, adjusted HR, 0.390; 95% CI, 0.139-1.095; p=0.074) did not differ between 2 stent groups after complex PCI.
CONCLUSIONS
Clinical outcomes of BP-BES were comparable to those of DP-EES at 2 years after complex PCI. Our data suggest that use of BP-BES is acceptable, even for complex PCI.
MeSH Terms
Figure
Cited by 1 articles
-
Stent Selection in Complex Coronary Interventions: Thinking Complex?
Pil Hyung Lee, Seung-Whan Lee
Korean Circ J. 2019;49(1):81-83. doi: 10.4070/kcj.2018.0285.
Reference
-
1. Park DW, Park SW. Stent thrombosis in the era of the drug-eluting stent. Korean Circ J. 2005; 35:791–794.
Article2. Minha S, Barbash IM, Dvir D, et al. Second-generation everolimus-eluting stents compared to first-generation drug-eluting stents in patients treated for multivessel disease. J Interv Cardiol. 2013; 26:561–569.
Article3. Grundeken MJ, Wykrzykowska JJ, Ishibashi Y, et al. First generation versus second generation drug-eluting stents for the treatment of bifurcations: 5-year follow-up of the LEADERS all-comers randomized trial. Catheter Cardiovasc Interv. 2016; 87:E248–60.
Article4. Vlachojannis GJ, Smits PC, Hofma SH, et al. Biodegradable polymer biolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with coronary artery disease: final 5-year report from the COMPARE II trial (abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent). JACC Cardiovasc Interv. 2017; 10:1215–1221.5. Im E, Kim GS, Shin DH, et al. Long-term clinical outcomes of a biodegradable polymer-based biolimus-eluting stent. J Interv Cardiol. 2016; 29:162–167.
Article6. Cavalcante R, Sotomi Y, Mancone M, et al. Impact of the SYNTAX scores I and II in patients with diabetes and multivessel coronary disease: a pooled analysis of patient level data from the SYNTAX, PRECOMBAT, and BEST trials. Eur Heart J. 2017; 38:1969–1977.
Article7. Hausleiter J, Kastrati A, Mehilli J, et al. Impact of lesion complexity on the capacity of a trial to detect differences in stent performance: results from the ISAR-STEREO trial. Am Heart J. 2003; 146:882–886.
Article8. Lee DH, Park TK, Song YB, et al. Clinical outcomes of biodegradable polymer biolimus-eluting BioMatrix stents versus durable polymer everolimus-eluting Xience stents. PLoS One. 2017; 12:e0183079.
Article9. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007; 115:2344–2351.10. Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011; 42:1–28.
Article11. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014; 33:1242–1258.
Article12. Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet. 2013; 381:651–660.
Article13. Kaiser C, Galatius S, Jeger R, et al. Long-term efficacy and safety of biodegradable-polymer biolimus-eluting stents: main results of the Basel Stent Kosten-Effektivitäts Trial-PROspective Validation Examination II (BASKET-PROVE II), a randomized, controlled noninferiority 2-year outcome trial. Circulation. 2015; 131:74–81.14. Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ. 2013; 347:f6625.
Article15. Navarese EP, Tandjung K, Claessen B, et al. Safety and efficacy outcomes of first and second generation durable polymer drug eluting stents and biodegradable polymer biolimus eluting stents in clinical practice: comprehensive network meta-analysis. BMJ. 2013; 347:f6530.
Article16. Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J. 2014; 35:1147–1158.
Article17. Sakurai R, Burazor I, Bonneau HN, Kaneda H. Long-term outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer everolimus-eluting stents: a meta-analysis of randomized controlled trials. Int J Cardiol. 2016; 223:1066–1071.
Article18. Grube E, Buellesfeld L. BioMatrix Biolimus A9-eluting coronary stent: a next-generation drug-eluting stent for coronary artery disease. Expert Rev Med Devices. 2006; 3:731–741.19. Pache J, Kastrati A, Mehilli J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003; 41:1283–1288.
Article20. Kolandaivelu K, Swaminathan R, Gibson WJ, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011; 123:1400–1409.
Article21. Sim DS, Jeong MH, Park DS, et al. Effect of pretreatment of ezetimibe/simvastatin on arterial healing and endothelialization after drug-eluting stent implantation in a porcine coronary restenosis model. Korean Circ J. 2015; 45:110–116.
Article22. Giustino G, Baber U, Aquino M, et al. Safety and efficacy of new-generation drug-eluting stents in women undergoing complex percutaneous coronary artery revascularization: from the win-des collaborative patient-level pooled analysis. JACC Cardiovasc Interv. 2016; 9:674–684.23. Migliorini A, Valenti R, Parodi G, et al. Angiographic and clinical outcomes after everolimus-eluting stenting for unprotected left main disease and high anatomic coronary complexity. JACC Cardiovasc Interv. 2016; 9:1001–1007.
Article24. Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012; 125:505–513.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrathin Bioresorbable Polymer Sirolimus-Eluting Orsiro Stents for Complex Percutaneous Coronary Intervention
- Histopathological Comparison among Biolimus, Zotarolimus and Everolimus-Eluting Stents in Porcine Coronary Restenosis Model
- Nobori-Biolimus-Eluting Stents versus Resolute Zotarolimus-Eluting Stents in Patients Undergoing Coronary Intervention: A Propensity Score Matching
- Coronary Stent Thrombosis: Current Insights into New Drug-Eluting Stent Designs
- A Comparative Analysis of Two Biolimus-Eluting Biodegradable Stents: Evaluation of the Equivalence of 6 Months Versus 12 Months of DAPT Following Biolimus-Eluting Stent Implantation in Acute Coronary Syndrome (EQUUS)